Biology:Ferroptosis
Ferroptosis (also known as oxytosis) is a type of programmed cell death dependent on iron and characterized by the accumulation of lipid peroxides. Ferroptosis is biochemically, genetically, and morphologically distinct from other forms of regulated cell death such as apoptosis and necroptosis.[1] Oxytosis/ferroptosis can be initiated by the failure of the glutathione-dependent antioxidant defenses, resulting in unchecked lipid peroxidation and eventual cell death.[2] Lipophilic antioxidants[3] and iron chelators[1] can prevent ferroptotic cell death.
Researchers have identified roles in which oxytosis/ferroptosis can contribute to the medical field, such as the development of cancer therapies.[4] Ferroptosis activation plays a regulatory role on growth of tumor cells in the human body. However, the positive effects of oxytosis/ferroptosis could be potentially neutralized by its disruption of metabolic pathways and disruption of homeostasis in the human body.[5] Since oxytosis/ferroptosis is a form of regulated cell death,[6] some of the molecules that regulate oxytosis/ferroptosis are involved in metabolic pathways that regulate cysteine exploitation, glutathione state, nicotinamide adenine dinucleotide phosphate (NADP) function, lipid peroxidation, and iron homeostasis.[5]
History
In 1989, work by the groups of Joseph T. Coyle and Ronald Schnaar showed in a neuronal cell line that excess exposure to glutamate or lowered cystine causes a decrease in glutathione levels, an accumulation in intracellular peroxides, and cytotoxicity.[7][8] Later work by Pamela Maher and David Schubert noted the distinction of this cell death process from apoptosis, describing it as oxidative glutamate toxicity or oxytosis.[9][10][11] In 2012, a study by Brent Stockwell and Scott Dixon characterized the iron dependence of this cell death process and coined the term ferroptosis.[1] Oxytosis and ferroptosis are now thought to be the same cell death mechanism.[12][13]
Other early studies regarding the connection between iron and lipid peroxidation,[14][15][16][17][18] cystine deprivation and oxidative cell death,[19][20][21][22][23] the activity and importance of glutathione peroxidase 4 (GPX4),[24][25][26][27][28] and the identification of small molecules that induce ferroptosis[29][30][31] were key to the eventual characterization of ferroptosis.
Mechanism
The hallmark feature of oxytosis/ferroptosis is the iron-dependent accumulation of oxidatively damaged phospholipids, i.e., lipid peroxides. The implication of Fenton chemistry via iron is crucial for the generation of reactive oxygen species and this feature can be exploited by sequestering iron in lysosomes.[32] Ferroptosis has been shown to involve distinct cellular organelles,[33] which includes peroxisomes,[34] mitochondria,[35] the endoplasmic reticulum (ER)[36] and lysosomes.[37][38][39] It has been a debate in the scientific community, where ferroptosis is initiated in the cell, and now research points to the lysosome,[40] where the chemical environment (iron, pH an hydrogen peroxide) are favorable. Oxidation of phospholipids can occur when free radicals abstract electrons from a lipid molecule (typically affecting polyunsaturated fatty acids), thereby promoting their oxidation.
The primary cellular mechanism of protection against oxytosis/ferroptosis is mediated by the selenoprotein GPX4, a glutathione-dependent hydroperoxidase that converts lipid peroxides into non-toxic lipid alcohols.[41][42] Recently, a second parallel protective pathway was independently discovered by two labs that involves the oxidoreductase FSP1 (also known as AIFM2).[43][44] FSP1 enzymatically reduces non-mitochondrial coenzyme Q10 (CoQ10), thereby generating a potent lipophilic antioxidant that suppresses the propagation of lipid peroxides.[43][44] Vitamin K is also reduced by FSP1 to a hydroquinone species that also acts as a radical-trapping antoxidant and suppressor of ferroptosis.[45] A similar mechanism for a cofactor moonlighting as a diffusable antioxidant was discovered in the same year for tetrahydrobiopterin (BH4), a product of the rate-limiting enzyme GTP cyclohdrolase 1 (GCH1).[46][47]
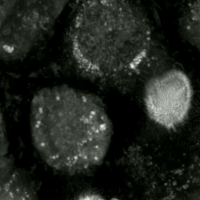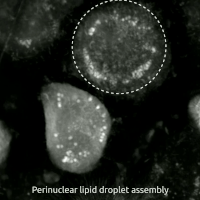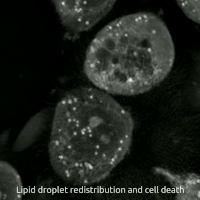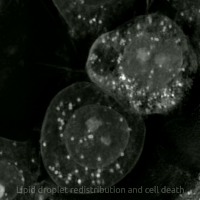
Replacing natural polyunsaturated fatty acids (PUFA) with deuterated PUFA (dPUFA), which have deuterium in place of the bis-allylic hydrogens, can prevent cell death induced by erastin or RSL3.[48] These deuterated PUFAs effectively inhibit ferroptosis and various chronic degenerative diseases associated with ferroptosis.[49]
Biology
Ferroptosis was initially characterized in human cell lines and has been since found to occur in other mammals (mice),[50] avians (chicken),[51] worms (C. elegans),[52][53] and plants (A. thaliana,[54][55][56] T. aestivum L.,[57] and others).[58][59][60][61] Ferroptosis has also been demonstrated in canine cancer cell models.[62] There have been limited studies in other model organisms such as D. melanogaster.[63][64] Elements related to components of the ferroptosis pathway have been identified in archaea, bacteria, and fungi, though it is unclear the extent to which ferroptosis occurs in these organisms.[65][66] Further studies in this area may reveal an ancient origin for ferroptosis.
Unlike other forms of cell death, ferroptosis has been shown to propagate between cells in a wave-like manner.[7][8] This phenomenon is promoted by secretion of galectin-13 during ferroptosis. Mechanistically, galectin-13 binds to CD44, inhibiting CD44-mediated membrane localization of SLC7A11.[9]
In development
During embryonic development, many cells die via apoptosis and other cell death pathways for various purposes including morphogenesis tissue sculpting, controlling cell numbers, and quality control.[67][68] In 2024, it was found that ferroptosis plays a role in normal physiology during embryonic development and muscle remodelling, propagating in millimeter-length waves through the developing avian limb.[51] The exact pro-ferroptotic signal that is transmitted between cells and the manner by which these ferroptotic waves are bounded remain to be characterized.
Therapeutic relevance

Fundamental discoveries uncovering the biology of ferroptosis and translational studies showing the disease relevance of ferroptosis have motivated efforts to develop therapeutics that modulate ferroptosis. For example, Kojin Therapeutics and PTC Therapeutics are exploring ferroptosis modulation for treatment of cancer and Friedrich's ataxia.[62][69][70][71] Ferroptosis has been implicated in a range of different diseases including cancer, ischemia/reperfusion injury (IRI), inflammation, neurodegeneration, and kidney injury.[72]
Cancer
Ferroptosis has been explored as a strategy to selectively kill cancer cells.[73]
- Breast
- Acute myeloid leukemia
- Pancreatic ductal adenocarcinoma
- Ovarian
- B-cell lymphoma
- Renal cell carcinomas
- Lung
- Glioblastoma
These forms of cancer have been hypothesized to be highly sensitive to oxytosis/ferroptosis induction. An upregulation of iron levels has also been seen to induce oxytosis/ferroptosis in certain types of cancer, such as breast cancer.[4] Breast cancer cells have exhibited vulnerability to oxytosis/ferroptosis via a combination of siramesine and lapatinib. These cells also exhibited an autophagic cycle independent of ferroptotic activity, indicating that the two different forms of cell death could be controlled to activate at specific times following treatment.[74] Furthermore, intratumor bacteria may scavenge iron by producing iron siderophores, which indirectly protect tumor cells from ferroptosis, emphasizing the need for ferroptosis inducers (thiostrepton) for cancer treatment.[75]
In various contexts, resistance to cancer therapy is associated with a mesenchymal state.[76][77][78] A pair of studies in 2017 found that these cancer cells in this therapy-induced drug-resistant state exhibit a greater dependence on GPX4 to suppress ferroptosis. Consequently, GPX4 inhibition represents a possible therapeutic strategy to mitigate acquired drug resistance.[79][80]
Neurodegeneration
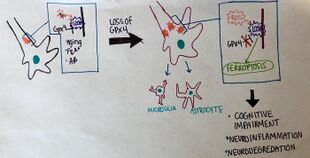
Neural connections are constantly changing within the nervous system. Synaptic connections that are used more often are kept intact and promoted, while synaptic connections that are rarely used are subject to degradation. Elevated levels of synaptic connection loss and degradation of neurons are linked to neurodegenerative diseases.[81] More recently, oxytosis/ferroptosis has been linked to diverse brain diseases,[82] in particular, Alzheimer's disease, amyotrophic lateral sclerosis (ALS), and Parkinson's disease.[83] Two new studies show that oxytosis/ferroptosis contributes to neuronal death after intracerebral hemorrhage.[84][85] Neurons that are degraded through oxytosis/ferroptosis release lipid metabolites from inside the cell body. The lipid metabolites are harmful to surrounding neurons, causing inflammation in the brain. Inflammation is a pathological feature of Alzheimer's disease and intracerebral hemorrhage. Recent studies have suggested that oxytosis/ferroptosis contributes to neuronal cell death after traumatic brain injury.[86] Hypoxic brain injury in resuscitated patients shows retrospective evidence of ferroptosis [87]. Additionally there is evidence that the prion protein and pathogenic prions PrPSc contribute to ferroptosis sensitivity in the brain, a condition that is enhanced by RAC3 expression. [88]
Acute kidney injury
Ferroptosis occurs during acute kidney injury in various cellular and animal models.[50][89][90][91][92] Deficiencies in ferroptosis suppressor enzymes such as GPX4 and FSP1 sensitize kidneys to tubular ferroptosis during kidney IRI, thus inhibition of ferroptosis may be of therapeutic benefit.[91] However, in premenopausal women this therapeutic potential might be limited due to intrinsic anti-ferroptotic effects of estrogen.[93]
During chemotherapy treatment, ferroptosis contributes to acute kidney injury.[94][95][96] Reagents to image ferroptosis have been developed to monitor anticancer drug-induced acute kidney injury in mouse models.[97]
Immunology
Ferroptosis has been implicated in many immune processes including both adaptive and innate immunity and diseases such as infection and autoimmune disease.[98][72][99][100]
Systemic lupus erythematosus
Systemic lupus erythematosus (SLE) is a chronic autoimmune disease.[101] Studies have implicated a role for neutrophil death (NETosis) in SLE.[102][103][104] Neutrophil ferroptosis is prevalent in patients with SLE and is induced by autoantibodies and interferon-alpha (IFN-α), which suppress GPX4 expression via the transcriptional repressor CREMα. Inhibition of ferroptosis was able to ameliorate SLE disease progression in the MRL/lpr mouse model of SLE.[105]
Inflammatory bowel diseases
There is a genetic association between GPX4 and Crohn's disease.[106] Subsequent study found that small intestinal epithelial cells (IECs) from Crohn's disease patient samples show reduced GPX4 expression and activity and lipid peroxidation.[107] The same study found that dietary lipids in Western diets such as the PUFA arachidonic acid can trigger enteritis resembling Crohn's disease in a mouse model.[107]
Biomarkers
Fatty acid-binding protein 5 (FABP5) has been reported as a stable biomarker of ferroptosis for retrospective pathological analyses. [108]
Small molecule modulators of ferroptosis

Inducers
Many compounds commonly used in ferroptosis studies including erastin,[29] RSL3 (RAS-selective lethal),[31] ML162, and ML210 [from National Institutes of Health-Molecular Libraries Small Molecule Repository (NIH-MLSMR)][109] were initially identified in screens for compounds that can selectively kill cancerous mutant RAS cells.
Initial studies characterized the mitochondrial VDAC2 and VDAC3 as the targets of erastin,[30] though it was later found that the mechanistic target of erastin is the cystine/glutamate transporter system xc−.[1] Erastin inhibits system xc−, lowering intracellular GSH levels.[1] Consequently, the GSH-dependent GPX4 is unable to detoxify lipid hydroperoxide species, leading to ferroptotic cell death. Derivatives of erastin have been prepared to improve aqueous solubility, potency, and metabolic stability, with imidazole ketone erastin (IKE) being the most extensively studied.[41][110][111]
RSL3 and ML162 contain chloroacetamide moieties that can covalently react with nucleophilic residues. RSL3 and ML162 are able to bind to and inhibit GPX4 enzymatic activity or degrade GPX4 in lysate-based assays,[80][41][112] though it has been found that RSL3 and ML162 do not inhibit purified GPX4 in vitro and target other selenoproteins such as thioredoxin reductase 1 (TXNRD1).[113] However, other TXNRD1 inhibitors do not trigger ferroptosis, suggesting that TXNRD1 inhibition is not sufficient to trigger ferroptosis.[113] The GPX4-inhibiting activity of RSL3 has also been suggested to be regulated by other factors such as 14-3-3ε[114] or through broad targeting of the selenoproteome.[115]
ML210 contains a nitroisoxazole group that acts as a masked nitrile-oxide electrophile. Specifically, in cellular and lysate contexts, ML210 undergoes ring-opening hydrolysis followed by a retro-Claisen-like condensation and ring-closing hydration to yield an unstable furoxan. Through a ring-opening tautomerization, this furoxan then yields a nitrile oxide that selectively reacts with selenocysteine residue 46 of GPX4.[116]


Upstream of GPX4, depletion of GSH by inhibiting GSH biosynthesis also induces ferroptosis. Work from Kojin Therapeutics and Ono Pharmaceutical has demonstrated that inhibition of glutamate-cysteine ligase (GCL), the rate-limiting enzyme in GSH biosynthesis, induces ferroptosis in cancer cell lines.[71][117] GCL also suppresses ferroptosis through a GSH-independent mechanisms such as limiting glutamate accumulation.[118] Buthionine sulfoximine (BSO) has been commonly used as a tool compound to inhibit GCL, though BSO is relatively low potency.[41][71][80] Accordingly, analogues have been reported that show improved potency and pharmacological properties that may be used in in vivo studies.[71][117]

FSP1 inhibition is generally not sufficient to induce ferroptosis but FSP1 inhibitors such as iFSP1 (targeting the CoQ10 binding site) and viFSP1 (versatile inhibitor of FSP1; targeting the NAD(P)H binding pocket) have been explored as ferroptosis sensitizers.[44][119][120][121] iFSP1 is not usable in rodent models, though viFSP1 is species-independent.[120] FSEN1 is an uncompetitive inhibitor of FSP1 that binds to the FSP1–NADH–CoQ complex.[119] 3-Phenylquinazolines (represented by icFSP1) do not competitively inhibit FSP1 enzymatic activity but rather trigger phase separation of FSP1 followed by induction of ferroptosis.[122] Notably, FSP1 activity can compensate for GPX4 loss and suppress ferroptosis in certain contexts.[43]
Inhibitors
Ferroptosis can be inhibited by lipophilic radical trapping antioxidants such as ferrostatin-1,[1][3] liproxstatin-1,[3] and vitamin E.[30] Chelation of iron by agents such as desferrioxamine mesylate (DFO) also prevents lipid peroxidation and suppresses ferroptosis.[31][123]
See also
References
- ↑ 1.0 1.1 1.2 1.3 1.4 1.5 "Ferroptosis: an iron-dependent form of nonapoptotic cell death". Cell 149 (5): 1060–1072. May 2012. doi:10.1016/j.cell.2012.03.042. PMID 22632970.
- ↑ "Mechanisms of ferroptosis". Cellular and Molecular Life Sciences 73 (11–12): 2195–2209. June 2016. doi:10.1007/s00018-016-2194-1. PMID 27048822.
- ↑ 3.0 3.1 3.2 "On the Mechanism of Cytoprotection by Ferrostatin-1 and Liproxstatin-1 and the Role of Lipid Peroxidation in Ferroptotic Cell Death". ACS Central Science 3 (3): 232–243. March 2017. doi:10.1021/acscentsci.7b00028. PMID 28386601.
- ↑ 4.0 4.1 "The Role of Ferroptosis in Cancer Development and Treatment Response". Frontiers in Pharmacology 8. 12 January 2018. doi:10.3389/fphar.2017.00992. PMID 29375387.
- ↑ 5.0 5.1 "Metabolic networks in ferroptosis". Oncology Letters 15 (4): 5405–5411. April 2018. doi:10.3892/ol.2018.8066. PMID 29556292.
- ↑ "Cell death mechanisms in eukaryotes". Cell Biology and Toxicology 36 (2): 145–164. April 2020. doi:10.1007/s10565-019-09496-2. PMID 31820165.
- ↑ 7.0 7.1 "Glutamate toxicity in a neuronal cell line involves inhibition of cystine transport leading to oxidative stress". Neuron 2 (6): 1547–1558. June 1989. doi:10.1016/0896-6273(89)90043-3. PMID 2576375.
- ↑ 8.0 8.1 "Immature cortical neurons are uniquely sensitive to glutamate toxicity by inhibition of cystine uptake". FASEB Journal 4 (6): 1624–1633. April 1990. doi:10.1096/fasebj.4.6.2180770. PMID 2180770.
- ↑ 9.0 9.1 "Oxidative glutamate toxicity can be a component of the excitotoxicity cascade". The Journal of Neuroscience 21 (19): 7455–7462. October 2001. doi:10.1523/JNEUROSCI.21-19-07455.2001. PMID 11567035.
- ↑ "Oxytosis: A novel form of programmed cell death". Current Topics in Medicinal Chemistry 1 (6): 497–506. December 2001. doi:10.2174/1568026013394741. PMID 11895126.
- ↑ "Oxidative stress induces a form of programmed cell death with characteristics of both apoptosis and necrosis in neuronal cells". Journal of Neurochemistry 71 (1): 95–105. July 1998. doi:10.1046/j.1471-4159.1998.71010095.x. PMID 9648855.
- ↑ "Oxytosis/Ferroptosis-(Re-) Emerging Roles for Oxidative Stress-Dependent Non-apoptotic Cell Death in Diseases of the Central Nervous System". Frontiers in Neuroscience 12. 2018. doi:10.3389/fnins.2018.00214. PMID 29731704.
- ↑ "Using the Oxytosis/Ferroptosis Pathway to Understand and Treat Age-Associated Neurodegenerative Diseases". Cell Chemical Biology 27 (12): 1456–1471. December 2020. doi:10.1016/j.chembiol.2020.10.010. PMID 33176157.
- ↑ "Lipid peroxidation initiated by superoxide-dependent hydroxyl radicals using complexed iron and hydrogen peroxide". FEBS Letters 172 (2): 245–249. July 1984. doi:10.1016/0014-5793(84)81134-5. PMID 6086389. Bibcode: 1984FEBSL.172..245G.
- ↑ "The requirement for iron (III) in the initiation of lipid peroxidation by iron (II) and hydrogen peroxide". The Journal of Biological Chemistry 262 (3): 1098–1104. January 1987. doi:10.1016/S0021-9258(19)75755-X. PMID 3027077.
- ↑ "The involvement of iron in lipid peroxidation. Importance of ferric to ferrous ratios in initiation". The Journal of Biological Chemistry 261 (22): 10282–10289. August 1986. doi:10.1016/S0021-9258(18)67521-0. PMID 3015924.
- ↑ "The role of iron in the initiation of lipid peroxidation". Chemistry and Physics of Lipids 44 (2–4): 191–208. 1987. doi:10.1016/0009-3084(87)90050-8. PMID 2822270.
- ↑ "Sources and role of iron in lipid peroxidation". Chemical Research in Toxicology 6 (2): 134–146. 1993. doi:10.1021/tx00032a001. PMID 8477003.
- ↑ "Nutrition needs of mammalian cells in tissue culture". Science 122 (3168): 501–514. September 1955. doi:10.1126/science.122.3168.501. PMID 13255879. Bibcode: 1955Sci...122..501E.
- ↑ "The biosynthesis of cystine in human cell cultures". The Journal of Biological Chemistry 236 (5): 1425–1428. May 1961. doi:10.1016/S0021-9258(18)64190-0. PMID 13725478.
- ↑ "Cystine deprivation induces oligodendroglial death: rescue by free radical scavengers and by a diffusible glial factor". Journal of Neurochemistry 67 (2): 566–573. August 1996. doi:10.1046/j.1471-4159.1996.67020566.x. PMID 8764581.
- ↑ "Macromolecular synthesis inhibitors prevent oxidative stress-induced apoptosis in embryonic cortical neurons by shunting cysteine from protein synthesis to glutathione". The Journal of Neuroscience 14 (7): 4385–4392. July 1994. doi:10.1523/JNEUROSCI.14-07-04385.1994. PMID 8027786.
- ↑ "Effect of antioxidants on cultured human diploid fibroblasts exposed to cystine-free medium". Biochemical and Biophysical Research Communications 74 (4): 1582–1588. February 1977. doi:10.1016/0006-291x(77)90623-4. PMID 843380. Bibcode: 1977BBRC...74.1582B.
- ↑ "Purification from pig liver of a protein which protects liposomes and biomembranes from peroxidative degradation and exhibits glutathione peroxidase activity on phosphatidylcholine hydroperoxides". Biochimica et Biophysica Acta (BBA) - Lipids and Lipid Metabolism 710 (2): 197–211. February 1982. doi:10.1016/0005-2760(82)90150-3. PMID 7066358.
- ↑ "The selenoenzyme phospholipid hydroperoxide glutathione peroxidase". Biochimica et Biophysica Acta (BBA) - General Subjects 839 (1): 62–70. March 1985. doi:10.1016/0304-4165(85)90182-5. PMID 3978121.
- ↑ "The selenoprotein GPX4 is essential for mouse development and protects from radiation and oxidative damage insults". Free Radical Biology & Medicine 34 (4): 496–502. February 2003. doi:10.1016/S0891-5849(02)01360-6. PMID 12566075.
- ↑ "Glutathione peroxidase 4 senses and translates oxidative stress into 12/15-lipoxygenase dependent- and AIF-mediated cell death". Cell Metabolism 8 (3): 237–248. September 2008. doi:10.1016/j.cmet.2008.07.005. PMID 18762024.
- ↑ "Cysteine mutant of mammalian GPx4 rescues cell death induced by disruption of the wild-type selenoenzyme". FASEB Journal 25 (7): 2135–2144. July 2011. doi:10.1096/fj.10-177147. PMID 21402720.
- ↑ 29.0 29.1 "Identification of genotype-selective antitumor agents using synthetic lethal chemical screening in engineered human tumor cells". Cancer Cell 3 (3): 285–296. March 2003. doi:10.1016/s1535-6108(03)00050-3. PMID 12676586.
- ↑ 30.0 30.1 30.2 "RAS-RAF-MEK-dependent oxidative cell death involving voltage-dependent anion channels". Nature 447 (7146): 864–868. June 2007. doi:10.1038/nature05859. PMID 17568748. Bibcode: 2007Natur.447..865Y.
- ↑ 31.0 31.1 31.2 "Synthetic lethal screening identifies compounds activating iron-dependent, nonapoptotic cell death in oncogenic-RAS-harboring cancer cells". Chemistry & Biology 15 (3): 234–245. March 2008. doi:10.1016/j.chembiol.2008.02.010. PMID 18355723.
- ↑ "Salinomycin kills cancer stem cells by sequestering iron in lysosomes". Nature Chemistry 9 (10): 1025–1033. October 2017. doi:10.1038/nchem.2778. PMID 28937680. Bibcode: 2017NatCh...9.1025M.
- ↑ Chen, Xin; Kang, Rui; Kroemer, Guido; Tang, Daolin (October 2021). "Organelle-specific regulation of ferroptosis" (in en). Cell Death & Differentiation 28 (10): 2843–2856. doi:10.1038/s41418-021-00859-z. ISSN 1476-5403. PMID 34465893.
- ↑ Zou, Yilong; Henry, Whitney S.; Ricq, Emily L.; Graham, Emily T.; Phadnis, Vaishnavi V.; Maretich, Pema; Paradkar, Sateja; Boehnke, Natalie et al. (September 2020). "Plasticity of ether lipids promotes ferroptosis susceptibility and evasion" (in en). Nature 585 (7826): 603–608. doi:10.1038/s41586-020-2732-8. ISSN 1476-4687. PMID 32939090. Bibcode: 2020Natur.585..603Z.
- ↑ Gao, Minghui; Yi, Junmei; Zhu, Jiajun; Minikes, Alexander M.; Monian, Prashant; Thompson, Craig B.; Jiang, Xuejun (2019-01-17). "Role of Mitochondria in Ferroptosis" (in English). Molecular Cell 73 (2): 354–363.e3. doi:10.1016/j.molcel.2018.10.042. ISSN 1097-2765. PMID 30581146.
- ↑ Dixon, Scott J; Patel, Darpan N; Welsch, Matthew; Skouta, Rachid; Lee, Eric D; Hayano, Miki; Thomas, Ajit G; Gleason, Caroline E et al. (2014-05-20). van der Donk, Wilfred. ed. "Pharmacological inhibition of cystine–glutamate exchange induces endoplasmic reticulum stress and ferroptosis". eLife 3. doi:10.7554/eLife.02523. ISSN 2050-084X. PMID 24844246.
- ↑ Mai, Trang Thi; Hamaï, Ahmed; Hienzsch, Antje; Cañeque, Tatiana; Müller, Sebastian; Wicinski, Julien; Cabaud, Olivier; Leroy, Christine et al. (October 2017). "Salinomycin kills cancer stem cells by sequestering iron in lysosomes" (in en). Nature Chemistry 9 (10): 1025–1033. doi:10.1038/nchem.2778. ISSN 1755-4349. PMID 28937680. Bibcode: 2017NatCh...9.1025M.
- ↑ Torii, Seiji; Shintoku, Ryosuke; Kubota, Chisato; Yaegashi, Makoto; Torii, Ryoko; Sasaki, Masaya; Suzuki, Toshinobu; Mori, Masanobu et al. (2016-03-10). "An essential role for functional lysosomes in ferroptosis of cancer cells". Biochemical Journal 473 (6): 769–777. doi:10.1042/BJ20150658. ISSN 0264-6021. PMID 26759376. https://portlandpress.com/biochemj/article-abstract/473/6/769/49342/An-essential-role-for-functional-lysosomes-in?redirectedFrom=fulltext.
- ↑ Cañeque, Tatiana; Baron, Leeroy; Müller, Sebastian; Carmona, Alanis; Colombeau, Ludovic; Versini, Antoine; Solier, Stéphanie; Gaillet, Christine et al. (2025-05-07). "Activation of lysosomal iron triggers ferroptosis in cancer" (in en). Nature 642 (8067): 492–500. doi:10.1038/s41586-025-08974-4. ISSN 1476-4687. PMID 40335696. Bibcode: 2025Natur.642..492C.
- ↑ Cañeque, Tatiana; Baron, Leeroy; Müller, Sebastian; Carmona, Alanis; Colombeau, Ludovic; Versini, Antoine; Solier, Stéphanie; Gaillet, Christine et al. (2025-05-07). "Activation of lysosomal iron triggers ferroptosis in cancer" (in en). Nature 642 (8067): 492–500. doi:10.1038/s41586-025-08974-4. ISSN 1476-4687. PMID 40335696. Bibcode: 2025Natur.642..492C.
- ↑ 41.0 41.1 41.2 41.3 "Regulation of ferroptotic cancer cell death by GPX4". Cell 156 (1–2): 317–331. January 2014. doi:10.1016/j.cell.2013.12.010. PMID 24439385.
- ↑ "Selenium Utilization by GPX4 Is Required to Prevent Hydroperoxide-Induced Ferroptosis". Cell 172 (3): 409–422.e21. January 2018. doi:10.1016/j.cell.2017.11.048. PMID 29290465.
- ↑ 43.0 43.1 43.2 "The CoQ oxidoreductase FSP1 acts parallel to GPX4 to inhibit ferroptosis". Nature 575 (7784): 688–692. November 2019. doi:10.1038/s41586-019-1705-2. PMID 31634900. Bibcode: 2019Natur.575..688B.
- ↑ 44.0 44.1 44.2 "FSP1 is a glutathione-independent ferroptosis suppressor". Nature 575 (7784): 693–698. November 2019. doi:10.1038/s41586-019-1707-0. PMID 31634899. Bibcode: 2019Natur.575..693D. https://orca.cardiff.ac.uk/id/eprint/126674/.
- ↑ "A non-canonical vitamin K cycle is a potent ferroptosis suppressor". Nature 608 (7924): 778–783. August 2022. doi:10.1038/s41586-022-05022-3. PMID 35922516. Bibcode: 2022Natur.608..778M.
- ↑ "GTP Cyclohydrolase 1/Tetrahydrobiopterin Counteract Ferroptosis through Lipid Remodeling". ACS Central Science 6 (1): 41–53. January 2020. doi:10.1021/acscentsci.9b01063. PMID 31989025.
- ↑ "Metabolic determinants of cancer cell sensitivity to canonical ferroptosis inducers". Nature Chemical Biology 16 (12): 1351–1360. December 2020. doi:10.1038/s41589-020-0613-y. PMID 32778843.
- ↑ "Lipid Metabolism Regulates Oxidative Stress and Ferroptosis in RAS-Driven Cancers: A Perspective on Cancer Progression and Therapy". Frontiers in Molecular Biosciences 8. 2021. doi:10.3389/fmolb.2021.706650. PMID 34485382.
- ↑ "Ferroptosis: mechanisms, biology and role in disease". Nature Reviews. Molecular Cell Biology 22 (4): 266–282. April 2021. doi:10.1038/s41580-020-00324-8. PMID 33495651.
- ↑ 50.0 50.1 "Inactivation of the ferroptosis regulator Gpx4 triggers acute renal failure in mice". Nature Cell Biology 16 (12): 1180–1191. December 2014. doi:10.1038/ncb3064. PMID 25402683.
- ↑ 51.0 51.1 "Emergence of large-scale cell death through ferroptotic trigger waves". Nature 631 (8021): 654–662. July 2024. doi:10.1038/s41586-024-07623-6. PMID 38987590. Bibcode: 2024Natur.631..654C.
- ↑ "Changes in ferrous iron and glutathione promote ferroptosis and frailty in aging Caenorhabditis elegans". eLife 9. July 2020. doi:10.7554/eLife.56580. PMID 32690135.
- ↑ "Dietary Lipids Induce Ferroptosis in Caenorhabditiselegans and Human Cancer Cells" (in English). Developmental Cell 54 (4): 447–454.e4. August 2020. doi:10.1016/j.devcel.2020.06.019. PMID 32652074.
- ↑ "Heat stress induces ferroptosis-like cell death in plants". The Journal of Cell Biology 216 (2): 463–476. February 2017. doi:10.1083/jcb.201605110. PMID 28100685.
- ↑ "New Target in an Old Enemy: Herbicide (R)-Dichlorprop Induces Ferroptosis-like Death in Plants". Journal of Agricultural and Food Chemistry 69 (27): 7554–7564. July 2021. doi:10.1021/acs.jafc.1c02102. PMID 34196530. Bibcode: 2021JAFC...69.7554L.
- ↑ Song, Wangyang; Xin, Shan; He, Meng; Pfeiffer, Susanne; Cao, Aiping; Li, Hongbin; Schick, Joel A.; Jin, Xiang (2021). "Evolutionary and functional analyses demonstrate conserved ferroptosis protection by Arabidopsis GPXs in mammalian cells" (in en). The FASEB Journal 35 (6): e21550. doi:10.1096/fj.202000856R. ISSN 1530-6860.
- ↑ "Transcriptome Analysis of Heat Shock Factor C2a Over-Expressing Wheat Roots Reveals Ferroptosis-like Cell Death in Heat Stress Recovery". International Journal of Molecular Sciences 24 (4): 3099. February 2023. doi:10.3390/ijms24043099. PMID 36834507.
- ↑ "Ferroptosis induced by the biocontrol agent Pythium oligandrum enhances soybean resistance to Phytophthora sojae". Environmental Microbiology 24 (12): 6267–6278. December 2022. doi:10.1111/1462-2920.16248. PMID 36250814. Bibcode: 2022EnvMi..24.6267C.
- ↑ "Rice iron storage protein ferritin 2 (OsFER2) positively regulates ferroptotic cell death and defense responses against Magnaporthe oryzae". Frontiers in Plant Science 13. 2022. doi:10.3389/fpls.2022.1019669. PMID 36352872. Bibcode: 2022FrPS...1319669N.
- ↑ "The NIN-Like Protein OsNLP2 Negatively Regulates Ferroptotic Cell Death and Immune Responses to Magnaporthe oryzae in Rice". Antioxidants 11 (9): 1795. September 2022. doi:10.3390/antiox11091795. PMID 36139868.
- ↑ "The potential role of acrolein in plant ferroptosis-like cell death". PLOS ONE 14 (12). 2019. doi:10.1371/journal.pone.0227278. PMID 31887216. Bibcode: 2019PLoSO..1427278H.
- ↑ 62.0 62.1 "Validation of ferroptosis in canine cancer cells to enable comparative oncology and translational medicine". bioRxiv. April 2024. doi:10.1101/2024.04.28.591561. PMID 38746359.
- ↑ "α-Synuclein Toxicity in Drosophila melanogaster Is Enhanced by the Presence of Iron: Implications for Parkinson's Disease". Antioxidants 12 (2): 261. January 2023. doi:10.3390/antiox12020261. PMID 36829820.
- ↑ "The Protective Role of Mitochondrial Ferritin on Erastin-Induced Ferroptosis". Frontiers in Aging Neuroscience 8: 308. 2016. doi:10.3389/fnagi.2016.00308. PMID 28066232.
- ↑ "Regulation of lipid peroxidation and ferroptosis in diverse species". Genes & Development 32 (9–10): 602–619. May 2018. doi:10.1101/gad.314674.118. PMID 29802123.
- ↑ "C-ferroptosis is an iron-dependent form of regulated cell death in cyanobacteria". The Journal of Cell Biology 221 (2). February 2022. doi:10.1083/jcb.201911005. PMID 34817556.
- ↑ "Death in embryonic systems". Science 154 (3749): 604–612. November 1966. doi:10.1126/science.154.3749.604. PMID 5332319. Bibcode: 1966Sci...154..604S.
- ↑ "Cell death in animal development". Development 147 (14). July 2020. doi:10.1242/dev.191882. PMID 32709690.
- ↑ "Our Pipeline". https://kojintx.com/.
- ↑ "OUR SCIENCE: Ferroptosis & Inflammation – Targeting oxidative stress and inflammation pathways to treat CNS diseases". https://www.ptcbio.com/our-science/ferroptosis-and-inflammation/.
- ↑ 71.0 71.1 71.2 71.3 (in en) The enzyme glutamate-cysteine ligase (GCL) is a target for ferroptosis induction in cancer, 2024-04-30, doi:10.1101/2024.04.28.591552, https://www.biorxiv.org/content/10.1101/2024.04.28.591552v1, retrieved 2024-12-27
- ↑ 72.0 72.1 "Ferroptosis in health and disease". Redox Biology 75. September 2024. doi:10.1016/j.redox.2024.103211. PMID 38908072.
- ↑ "Targeting ferroptosis as a vulnerability in cancer". Nature Reviews. Cancer 22 (7): 381–396. July 2022. doi:10.1038/s41568-022-00459-0. PMID 35338310.
- ↑ "Ferroptosis and autophagy induced cell death occur independently after siramesine and lapatinib treatment in breast cancer cells". PLOS ONE 12 (8). 2017. doi:10.1371/journal.pone.0182921. PMID 28827805. Bibcode: 2017PLoSO..1282921M.
- ↑ "Bacterial Iron Siderophore Drives Tumor Survival and Ferroptosis Resistance in a Biofilm-Tumor Spheroid Coculture Model". Advanced Science 11 (39). October 2024. doi:10.1002/advs.202404467. PMID 39135304. Bibcode: 2024AdvSc..1104467Y.
- ↑ "Epithelial-to-mesenchymal transition is dispensable for metastasis but induces chemoresistance in pancreatic cancer". Nature 527 (7579): 525–530. November 2015. doi:10.1038/nature16064. PMID 26560028. Bibcode: 2015Natur.527..525Z.
- ↑ "Epithelial-to-mesenchymal transition is not required for lung metastasis but contributes to chemoresistance". Nature 527 (7579): 472–476. November 2015. doi:10.1038/nature15748. PMID 26560033. Bibcode: 2015Natur.527..472F.
- ↑ "EMT, CSCs, and drug resistance: the mechanistic link and clinical implications". Nature Reviews. Clinical Oncology 14 (10): 611–629. October 2017. doi:10.1038/nrclinonc.2017.44. PMID 28397828.
- ↑ "Drug-tolerant persister cancer cells are vulnerable to GPX4 inhibition". Nature 551 (7679): 247–250. November 2017. doi:10.1038/nature24297. PMID 29088702. Bibcode: 2017Natur.551..247H.
- ↑ 80.0 80.1 80.2 "Dependency of a therapy-resistant state of cancer cells on a lipid peroxidase pathway". Nature 547 (7664): 453–457. July 2017. doi:10.1038/nature23007. PMID 28678785.
- ↑ "Ablation of ferroptosis regulator glutathione peroxidase 4 in forebrain neurons promotes cognitive impairment and neurodegeneration". Redox Biology 12: 8–17. August 2017. doi:10.1016/j.redox.2017.01.021. PMID 28212525.
- ↑ "Ferroptosis and Its Role in Diverse Brain Diseases". Molecular Neurobiology 56 (7): 4880–4893. July 2019. doi:10.1007/s12035-018-1403-3. PMID 30406908.
- ↑ "Therapeutic inhibition of ferroptosis in neurodegenerative disease". Trends in Pharmacological Sciences 44 (10): 674–688. October 2023. doi:10.1016/j.tips.2023.07.007. PMID 37657967.
- ↑ "Inhibition of neuronal ferroptosis protects hemorrhagic brain". JCI Insight 2 (7). April 2017. doi:10.1172/jci.insight.90777. PMID 28405617.
- ↑ "Ultrastructural Characteristics of Neuronal Death and White Matter Injury in Mouse Brain Tissues After Intracerebral Hemorrhage: Coexistence of Ferroptosis, Autophagy, and Necrosis". Frontiers in Neurology 9. July 2018. doi:10.3389/fneur.2018.00581. PMID 30065697.
- ↑ "Traumatic Brain Injury: Ultrastructural Features in Neuronal Ferroptosis, Glial Cell Activation and Polarization, and Blood-Brain Barrier Breakdown". Cells 10 (5): 1009. April 2021. doi:10.3390/cells10051009. PMID 33923370.
- ↑ Peng, Hao; Xin, Shan; Pfeiffer, Susanne; Müller, Constanze; Merl-Pham, Juliane; Hauck, Stefanie M.; Harter, Patrick N.; Spitzer, Daniel et al. (2024-04-23). "Fatty acid-binding protein 5 is a functional biomarker and indicator of ferroptosis in cerebral hypoxia" (in en). Cell Death & Disease 15 (4): 286. doi:10.1038/s41419-024-06681-y. ISSN 2041-4889. PMID 38653992.
- ↑ Peng, Hao; Pfeiffer, Susanne; Varynskyi, Borys; Qiu, Marina; Srinark, Chanikarn; Jin, Xiang; Zhang, Xin; Williams, Katie et al. (2025-06-25). "Prion-induced ferroptosis is facilitated by RAC3" (in en). Nature Communications 16 (1): 5385. doi:10.1038/s41467-025-60793-3. ISSN 2041-1723. PMID 40562790. Bibcode: 2025NatCo..16.5385P.
- ↑ "Ferrostatins inhibit oxidative lipid damage and cell death in diverse disease models". Journal of the American Chemical Society 136 (12): 4551–4556. March 2014. doi:10.1021/ja411006a. PMID 24592866. Bibcode: 2014JAChS.136.4551S.
- ↑ "Synchronized renal tubular cell death involves ferroptosis". Proceedings of the National Academy of Sciences of the United States of America 111 (47): 16836–16841. November 2014. doi:10.1073/pnas.1415518111. PMID 25385600. Bibcode: 2014PNAS..11116836L.
- ↑ 91.0 91.1 "Dysfunction of the key ferroptosis-surveilling systems hypersensitizes mice to tubular necrosis during acute kidney injury". Nature Communications 12 (1). July 2021. doi:10.1038/s41467-021-24712-6. PMID 34285231. Bibcode: 2021NatCo..12.4402T.
- ↑ "ACSL4 deficiency confers protection against ferroptosis-mediated acute kidney injury". Redox Biology 51. May 2022. doi:10.1016/j.redox.2022.102262. PMID 35180475.
- ↑ Tonnus, Wulf; Maremonti, Francesca; Gavali, Shubhangi; Schlecht, Marlena Nastassja; Gembardt, Florian; Belavgeni, Alexia; Leinung, Nadja; Flade, Karolin et al. (2025-08-13). "Multiple oestradiol functions inhibit ferroptosis and acute kidney injury" (in en). Nature 645 (8082): 1011–1019. doi:10.1038/s41586-025-09389-x. ISSN 1476-4687. PMID 40804518. Bibcode: 2025Natur.645.1011T.
- ↑ "Mitochondria-dependent ferroptosis plays a pivotal role in doxorubicin cardiotoxicity". JCI Insight 5 (9): e132747, 132747. May 2020. doi:10.1172/jci.insight.132747. PMID 32376803.
- ↑ "Drugs Repurposed as Antiferroptosis Agents Suppress Organ Damage, Including AKI, by Functioning as Lipid Peroxyl Radical Scavengers". Journal of the American Society of Nephrology 31 (2): 280–296. February 2020. doi:10.1681/ASN.2019060570. PMID 31767624.
- ↑ "Role of ferroptosis in cisplatin-induced acute nephrotoxicity in mice". Journal of Trace Elements in Medicine and Biology 67. September 2021. doi:10.1016/j.jtemb.2021.126798. PMID 34087581. Bibcode: 2021JTEMB..6726798I.
- ↑ "Ferroptosis MRI for early detection of anticancer drug-induced acute cardiac/kidney injuries". Science Advances 9 (10). March 2023. doi:10.1126/sciadv.add8539. PMID 36888714. Bibcode: 2023SciA....9D8539Z.
- ↑ Gavali, Shubhangi; Maremonti, Francesca; Linkermann, Andreas (2024-03-21). "Cholesterol business: life or death by rust" (in en). Signal Transduction and Targeted Therapy 9 (1). doi:10.1038/s41392-024-01802-7. ISSN 2059-3635. PMID 38514620.
- ↑ "Ferroptosis: A Critical Moderator in the Life Cycle of Immune Cells". Frontiers in Immunology 13. 2022-05-10. doi:10.3389/fimmu.2022.877634. PMID 35619718.
- ↑ "Ferroptosis in infection, inflammation, and immunity". The Journal of Experimental Medicine 218 (6). June 2021. doi:10.1084/jem.20210518. PMID 33978684.
- ↑ "Systemic lupus erythematosus". Nature Reviews. Disease Primers 2 (1). June 2016. doi:10.1038/nrdp.2016.39. PMID 27306639.
- ↑ "Neutrophils activate plasmacytoid dendritic cells by releasing self-DNA-peptide complexes in systemic lupus erythematosus". Science Translational Medicine 3 (73): 73ra19. March 2011. doi:10.1126/scitranslmed.3001180. PMID 21389263.
- ↑ "Netting neutrophils are major inducers of type I IFN production in pediatric systemic lupus erythematosus". Science Translational Medicine 3 (73): 73ra20. March 2011. doi:10.1126/scitranslmed.3001201. PMID 21389264.
- ↑ "Neutrophil extracellular traps enriched in oxidized mitochondrial DNA are interferogenic and contribute to lupus-like disease". Nature Medicine 22 (2): 146–153. February 2016. doi:10.1038/nm.4027. PMID 26779811.
- ↑ "Glutathione peroxidase 4-regulated neutrophil ferroptosis induces systemic autoimmunity". Nature Immunology 22 (9): 1107–1117. September 2021. doi:10.1038/s41590-021-00993-3. PMID 34385713.
- ↑ "Host-microbe interactions have shaped the genetic architecture of inflammatory bowel disease". Nature 491 (7422): 119–124. November 2012. doi:10.1038/nature11582. PMID 23128233. Bibcode: 2012Natur.491..119..
- ↑ 107.0 107.1 "Dietary lipids fuel GPX4-restricted enteritis resembling Crohn's disease". Nature Communications 11 (1). April 2020. doi:10.1038/s41467-020-15646-6. PMID 32286299. Bibcode: 2020NatCo..11.1775M.
- ↑ Peng, Hao; Xin, Shan; Pfeiffer, Susanne; Müller, Constanze; Merl-Pham, Juliane; Hauck, Stefanie M.; Harter, Patrick N.; Spitzer, Daniel et al. (2024-04-23). "Fatty acid-binding protein 5 is a functional biomarker and indicator of ferroptosis in cerebral hypoxia" (in en). Cell Death & Disease 15 (4): 286. doi:10.1038/s41419-024-06681-y. ISSN 2041-4889. PMID 38653992.
- ↑ "Development of small-molecule probes that selectively kill cells induced to express mutant RAS". Bioorganic & Medicinal Chemistry Letters 22 (4): 1822–1826. February 2012. doi:10.1016/j.bmcl.2011.09.047. PMID 22297109.
- ↑ "Incorporation of metabolically stable ketones into a small molecule probe to increase potency and water solubility". Bioorganic & Medicinal Chemistry Letters 25 (21): 4787–4792. November 2015. doi:10.1016/j.bmcl.2015.07.018. PMID 26231156.
- ↑ "Imidazole Ketone Erastin Induces Ferroptosis and Slows Tumor Growth in a Mouse Lymphoma Model" (in English). Cell Chemical Biology 26 (5): 623–633.e9. May 2019. doi:10.1016/j.chembiol.2019.01.008. PMID 30799221.
- ↑ "Small-molecule allosteric inhibitors of GPX4". Cell Chemical Biology 29 (12): 1680–1693.e9. December 2022. doi:10.1016/j.chembiol.2022.11.003. PMID 36423641.
- ↑ 113.0 113.1 "The ferroptosis inducing compounds RSL3 and ML162 are not direct inhibitors of GPX4 but of TXNRD1". Redox Biology 62. June 2023. doi:10.1016/j.redox.2023.102703. PMID 37087975.
- ↑ "Inactivation of the glutathione peroxidase GPx4 by the ferroptosis-inducing molecule RSL3 requires the adaptor protein 14-3-3ε". FEBS Letters 594 (4): 611–624. February 2020. doi:10.1002/1873-3468.13631. PMID 31581313.
- ↑ "Recharacterization of RSL3 reveals that the selenoproteome is a druggable target in colorectal cancer". bioRxiv. August 2024. doi:10.1101/2024.03.29.587381. PMID 38617233.
- ↑ 116.0 116.1 "Selective covalent targeting of GPX4 using masked nitrile-oxide electrophiles". Nature Chemical Biology 16 (5): 497–506. May 2020. doi:10.1038/s41589-020-0501-5. PMID 32231343.
- ↑ 117.0 117.1 , 拓也; 周平 梅村 & 隆之 犬飼 et al."Gcl inhibitor" patent WO2023085367A1, issued 2023-05-19
- ↑ Kang, Yun Pyo; Mockabee-Macias, Andrea; Jiang, Chang; Falzone, Aimee; Prieto-Farigua, Nicolas; Stone, Everett; Harris, Isaac S.; DeNicola, Gina M. (2020-12-22). "Non-canonical glutamate-cysteine ligase activity protects against ferroptosis" (in en). Cell Metabolism 33 (1): 174–189.e7. doi:10.1016/j.cmet.2020.12.007. PMID 33357455.
- ↑ 119.0 119.1 "Identification of structurally diverse FSP1 inhibitors that sensitize cancer cells to ferroptosis". Cell Chemical Biology 30 (9): 1090–1103.e7. September 2023. doi:10.1016/j.chembiol.2023.04.007. PMID 37178691.
- ↑ 120.0 120.1 "Integrated chemical and genetic screens unveil FSP1 mechanisms of ferroptosis regulation". Nature Structural & Molecular Biology 30 (11): 1806–1815. November 2023. doi:10.1038/s41594-023-01136-y. PMID 37957306.
- ↑ "Identification of a Small Molecule That Enhances Ferroptosis via Inhibition of Ferroptosis Suppressor Protein 1 (FSP1)". ACS Chemical Biology 17 (2): 483–491. February 2022. doi:10.1021/acschembio.2c00028. PMID 35128925.
- ↑ "Phase separation of FSP1 promotes ferroptosis". Nature 619 (7969): 371–377. July 2023. doi:10.1038/s41586-023-06255-6. PMID 37380771. Bibcode: 2023Natur.619..371N.
- ↑ "Targeting Labile Iron-Mediated Ferroptosis Provides a Potential Therapeutic Strategy for Rhabdomyolysis-Induced Acute Kidney Injury". ACS Chemical Biology 18 (6): 1294–1304. June 2023. doi:10.1021/acschembio.2c00914. PMID 37172039.
External links
 |
