Biology:Fibrin
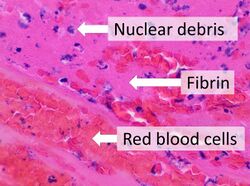

Fibrin (also called Factor Ia) is a fibrous, non-globular protein involved in the clotting of blood. It is formed by the action of the protease thrombin on fibrinogen, which causes it to polymerize. The polymerized fibrin, together with platelets, forms a hemostatic plug or clot over a wound site.
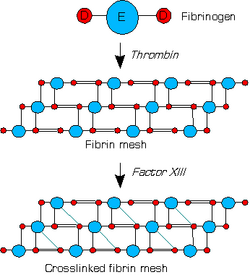
When the lining of a blood vessel is broken, platelets are attracted, forming a platelet plug. These platelets have thrombin receptors on their surfaces that bind serum thrombin molecules,[1] which in turn convert soluble fibrinogen in the serum into fibrin at the wound site. Fibrin forms long strands of tough insoluble protein that are bound to the platelets. Factor XIII completes the cross-linking of fibrin so that it hardens and contracts. The cross-linked fibrin forms a mesh atop the platelet plug that completes the clot. Fibrin was discovered[2] by Marcello Malpighi in 1666.[3]
Role in disease
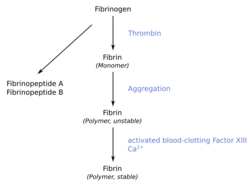
Excessive generation of fibrin due to activation of the coagulation cascade leads to thrombosis, the blockage of a vessel by an agglutination of red blood cells, platelets, polymerized fibrin and other components. Ineffective generation or premature lysis of fibrin increases the likelihood of a hemorrhage.
Dysfunction or disease of the liver can lead to a decrease in the production of fibrin's inactive precursor, fibrinogen, or to the production of abnormal fibrinogen molecules with reduced activity (dysfibrinogenaemia). Hereditary abnormalities of fibrinogen (the gene is carried on chromosome 4) are both quantitative and qualitative in nature and include afibrinogenaemia, hypofibrinogenaemia, dysfibrinogenaemia, and hypodysfibrinogenemia.
Reduced, absent, or dysfunctional fibrin is likely to render patients as hemophiliacs.
Physiology
Fibrin from various different animal sources is generally glycosylated with complex type biantennary asparagine-linked glycans. Variety is found in the degree of core fucosylation and in the type of sialic acid and galactose linkage.[4]
Structure
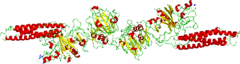
Fibrin is formed after thrombin cleavage of fibrinopeptide A (FPA) from fibrinogen Aalpha-chains, thus initiating fibrin polymerization. Double-stranded fibrils form through end-to-middle domain (D:E) associations, and concomitant lateral fibril associations and branching create a clot network.[5][6] Fibrin assembly facilitates intermolecular antiparallel C-terminal alignment of gamma-chain pairs, which are then covalently 'cross-linked' by factor XIII ('plasma protransglutaminase') or XIIIa to form 'gamma-dimers'. The image at the left is a crystal structure of the double-d fragment from human fibrin with two bound ligands. The experimental method used to obtain the image was X-ray diffraction, and it has a resolution of 2.30 Å. The structure is mainly made up of single alpha helices shown in red and beta sheets shown in yellow. The two blue structures are the bound ligands. The chemical structures of the ligands are Ca2+ ion, alpha-D-mannose (C6H12O6), and D-glucosamine (C6H13NO5).[7]
See also
- D-dimer
- Fibrin glue
- Fibrin scaffold
- Fibrinolysis
References
- ↑ Kehrel BE (2003). "[Blood platelets: biochemistry and physiology]" (in German). Hamostaseologie 23 (4): 149–158. doi:10.1055/s-0037-1619592. PMID 14603379.
- ↑ Arney, Kat (31 May 2017). "Fibrin and fibrinogen". Cambridge, UK: Royal Society of Chemistry. https://www.chemistryworld.com/podcasts/fibrin-and-fibrinogen/3007466.article.
- ↑ "350th Anniversary of the Discovery of Fibrin (1666–2016) History of Fibrin(ogen)". Winston-Salem: International Fibrinogen Research Society. 23 June 2016. https://www.fibrinogen.org/blog/350th-anniversary-of-the-discovery-of-fibrin-1666-2016-history.
- ↑ "Mass + retention time = structure: a strategy for the analysis of N-glycans by carbon LC-ESI-MS and its application to fibrin N-glycans". Anal. Chem. 79 (13): 5051–7. July 2007. doi:10.1021/ac070363i. PMID 17539604.
- ↑ Mosesson, M. W. (August 2005). "Fibrinogen and fibrin structure and functions". Journal of Thrombosis and Haemostasis 3 (8): 1894–1904. doi:10.1111/j.1538-7836.2005.01365.x. ISSN 1538-7933. PMID 16102057. https://pubmed.ncbi.nlm.nih.gov/16102057/.
- ↑ Undas, Anetta; Ariëns, Robert A.S. (2011-12-01). "Fibrin Clot Structure and Function". Arteriosclerosis, Thrombosis, and Vascular Biology 31 (12): e88–e99. doi:10.1161/ATVBAHA.111.230631. PMID 21836064. https://www.ahajournals.org/doi/10.1161/atvbaha.111.230631.
- ↑ Weisel, John W.; Litvinov, Rustem I. (2017), Parry, David A.D.; Squire, John M., eds., "Fibrin Formation, Structure and Properties", Fibrous Proteins: Structures and Mechanisms (Cham: Springer International Publishing) 82: pp. 405–456, doi:10.1007/978-3-319-49674-0_13, ISBN 978-3-319-49672-6, PMID 28101869
External links
- TGW1916.net, Defibrinated blood harvested from sheep (video)
- Fibrin: Molecule of the Month , by David Goodsell, RCSB Protein Data Bank
 |
