Chemistry:Ceruloplasmin
Ceruloplasmin (or caeruloplasmin) is a ferroxidase enzyme that in humans is encoded by the CP gene.[1][2][3]
Ceruloplasmin is the major copper-carrying protein in the blood, and in addition plays a role in iron metabolism. It was first described in 1948.[4] Another protein, hephaestin, is noted for its homology to ceruloplasmin, and also participates in iron and probably copper metabolism.
Function
Ceruloplasmin (CP) is an enzyme (EC 1.16.3.1) synthesized in the liver containing 6 atoms of copper in its structure.[5] Ceruloplasmin carries more than 95% of the total copper in healthy human plasma.[6] The rest is accounted for by macroglobulins. Ceruloplasmin exhibits a copper-dependent oxidase activity, which is associated with possible oxidation of Fe2+ (ferrous iron) into Fe3+ (ferric iron), therefore assisting in its transport in the plasma in association with transferrin, which can carry iron only in the ferric state.[7] The molecular weight of human ceruloplasmin is reported to be 151kDa.
Despite extensive research, much is still unknown about the exact functions of CP, most of the functions are attributed to CP focus on the presence of the Cu centers. These include copper transport to deliver the Cu to extrahepatic tissues, amine oxidase activity that controls the level of biogenic amines in intestinal fluids and plasma, removal of oxygen and other free radicals from plasma, and the export of iron from cells for transport through transferrin.[8]
Mutations have been known to disrupt the binding of copper to CP and will disrupt iron metabolism and cause an iron overload.
Ceruloplasmin is a relatively large enzyme (~10nm); the larger size prevents the bound copper from being lost in a person's urine during transport.
Active site structure
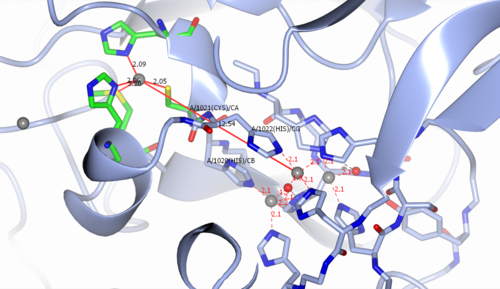
The multicopper active site of CP contains a type I (T1) mononuclear copper[8] site and a trinuclear copper center ~ 12-13 Å away (see figure 2). The tricopper center consists of two type III (T3) coppers and one type II (T2) copper ion. The two T3 copper ions are bridged by a hydroxide ligand while another hydroxide ligand links the T2 copper ion to the protein. The T1 center is bridged to the tricopper center by two histidine (His1020, His1022) residues and one Cys(1021) residue. The substrate binds near the T1 center and is oxidized by the T1 Cu2+ ion forming the reduced Cu+ oxidation state. The reduced T1 Cu+ then transfers the electron through the one Cys and two His bridging residues to the tricopper center. After four electrons have been transferred from the substrates to the copper centers, an O2 binds at the tricopper center and undergoes a four-electron reduction to form two molecules of water.[8]
Regulation
A cis-regulatory element called the GAIT element is involved in the selective translational silencing of the Ceruloplasmin transcript.[9] The silencing requires binding of a cytosolic inhibitor complex called IFN-gamma-activated inhibitor of translation (GAIT) to the GAIT element.[10]
Clinical significance
Like any other plasma protein, levels drop in patients with hepatic disease due to reduced synthesizing capabilities.
Mechanisms of low ceruloplasmin levels:
- Gene expression genetically low (aceruloplasminemia)
- Copper levels are low in general
- Malnutrition/trace metal deficiency in the food source
- Zinc toxicity, due to induced copper deficiency
- Copper does not cross the intestinal barrier due to ATP7A deficiency (Menkes disease and Occipital horn syndrome)
- Delivery of copper into the lumen of the ER-Golgi network is absent in hepatocytes due to absent ATP7B (Wilson's disease)
Copper availability doesn't affect the translation of the nascent protein. However, the apoenzyme without copper is unstable. Apoceruloplasmin is largely degraded intracellularly in the hepatocyte and the small amount that is released has a short circulation half life of 5 hours as compared to the 5.5 days for the holo-ceruloplasmin.
Ceruloplasmin can be measured by means of a blood test;[11] this can be done using immunoassays . The sample is spun and separated; it is stored around 4°C Celsius for three days. This test is to determine if there are signs of Wilson disease. Another test that can be done is a urine copper level test; this has been found to be less accurate than the blood test. A liver tissue test can be done as well.
Mutations in the ceruloplasmin gene (CP), which are very rare, can lead to the genetic disease aceruloplasminemia, characterized by hyperferritinemia with iron overload. In the brain, this iron overload may lead to characteristic neurologic signs and symptoms, such as cerebellar ataxia, progressive dementia, and extrapyramidal signs. Excess iron may also deposit in the liver, pancreas, and retina, leading to cirrhosis, endocrine abnormalities, and loss of vision, respectively.
Deficiency
Lower-than-normal ceruloplasmin levels may indicate the following:
- Wilson disease (a rare [UK incidence 2/100,000] copper storage disease).[12]
- Menkes disease (Menkes kinky hair syndrome) (rare – UK incidence 1/100,000)
- Copper deficiency
- Aceruloplasminemia[13]
- Zinc toxicity
Excess
Greater-than-normal ceruloplasmin levels may indicate or be noticed in:
- copper toxicity / zinc deficiency
- pregnancy
- oral contraceptive pill use[14]
- lymphoma
- acute and chronic inflammation (it is an acute-phase reactant)
- rheumatoid arthritis
- Angina[15]
- Alzheimer's disease[16]
- Schizophrenia[17]
- Obsessive-compulsive disorder[18]
Reference ranges
Normal blood concentration of ceruloplasmin in humans is 20–50 mg/dL.

References
- ↑ "Single-chain structure of human ceruloplasmin: the complete amino acid sequence of the whole molecule". Proceedings of the National Academy of Sciences of the United States of America 81 (2): 390–4. Jan 1984. doi:10.1073/pnas.81.2.390. PMID 6582496. Bibcode: 1984PNAS...81..390T.
- ↑ "Complete cDNA sequence of human preceruloplasmin". Proceedings of the National Academy of Sciences of the United States of America 83 (14): 5086–90. Jul 1986. doi:10.1073/pnas.83.14.5086. PMID 2873574. Bibcode: 1986PNAS...83.5086K.
- ↑ "Human genes encoding prothrombin and ceruloplasmin map to 11p11-q12 and 3q21-24, respectively". Somatic Cell and Molecular Genetics 13 (3): 285–92. May 1987. doi:10.1007/BF01535211. PMID 3474786.
- ↑ "Investigations in serum copper. II. Isolation of the Copper containing protein, and a description of its properties". Acta Chem Scand 2: 550–56. 1948. doi:10.3891/acta.chem.scand.02-0550.
- ↑ Endogenous Toxins: Targets for Disease Treatment and Prevention, 2 Volume Set. John Wiley & Sons. 2009. pp. 405–6. ISBN 978-3-527-32363-0. https://books.google.com/books?id=UaLR0RSuXvsC&pg=PA405.
- ↑ "Ceruloplasmin metabolism and function". Annual Review of Nutrition 22: 439–58. 2002. doi:10.1146/annurev.nutr.22.012502.114457. PMID 12055353.
- ↑ "Retinal iron homeostasis in health and disease". Frontiers in Aging Neuroscience 5: 24. 2013. doi:10.3389/fnagi.2013.00024. PMID 23825457.
- ↑ Jump up to: 8.0 8.1 8.2 Bertini, Ivano (2007) (in English). Biological Inorganic Chemistry. California, USA: University Science Books. pp. 426–442. ISBN 978-1-891389-43-6.
- ↑ "Transcript-selective translational silencing by gamma interferon is directed by a novel structural element in the ceruloplasmin mRNA 3' untranslated region". Molecular and Cellular Biology 23 (5): 1509–19. Mar 2003. doi:10.1128/MCB.23.5.1509-1519.2003. PMID 12588972.
- ↑ "Regulation of macrophage ceruloplasmin gene expression: one paradigm of 3'-UTR-mediated translational control". Molecules and Cells 20 (2): 167–72. Oct 2005. doi:10.1016/S1016-8478(23)13213-4. PMID 16267389.
- ↑ "Ceruloplasmin Test: MedlinePlus Medical Test" (in en). https://medlineplus.gov/lab-tests/ceruloplasmin-test/.
- ↑ "Deficiency of ceruloplasmin in patients with hepatolenticular degeneration (Wilson's disease)". Science 116 (3018): 484–5. Oct 1952. doi:10.1126/science.116.3018.484. PMID 12994898. Bibcode: 1952Sci...116..484S.
- ↑ "Aceruloplasminemia". Pediatric Research 44 (3): 271–6. Sep 1998. doi:10.1203/00006450-199809000-00001. PMID 9727700.
- ↑ "Green plasma-revisited". Anesthesiology 108 (4): 764–5. Apr 2008. doi:10.1097/ALN.0b013e3181672668. PMID 18362615.
- ↑ "Ceruloplasmin is a better predictor of the long-term prognosis compared with fibrinogen, CRP, and IL-6 in patients with severe unstable angina". Angiology 60 (1): 50–9. 2009. doi:10.1177/0003319708314249. PMID 18388036.
- ↑ "Cellular multitasking: the dual role of human Cu-ATPases in cofactor delivery and intracellular copper balance". Archives of Biochemistry and Biophysics 476 (1): 22–32. Aug 2008. doi:10.1016/j.abb.2008.05.005. PMID 18534184.
- ↑ "Plasma copper, iron, ceruloplasmin and ferroxidase activity in schizophrenia". Schizophrenia Research 86 (1–3): 167–71. Sep 2006. doi:10.1016/j.schres.2006.05.027. PMID 16842975.
- ↑ "High ceruloplasmin levels are associated with obsessive compulsive disorder: a case control study". Behavioral and Brain Functions 4: 52. 2008. doi:10.1186/1744-9081-4-52. PMID 19017404.
Further reading
- "Ceruloplasmin metabolism and function". Annual Review of Nutrition 22: 439–58. 2002. doi:10.1146/annurev.nutr.22.012502.114457. PMID 12055353.
- "Translational control by the 3'-UTR: the ends specify the means". Trends in Biochemical Sciences 28 (2): 91–8. Feb 2003. doi:10.1016/S0968-0004(03)00002-1. PMID 12575997.
- "Ceruloplasmin - acute-phase reactant or endogenous antioxidant? The case of cardiovascular disease". Medical Science Monitor 11 (2): RA48-51. Feb 2005. PMID 15668644.
- "Chemical evidence that proteolytic cleavage causes the heterogeneity present in human ceruloplasmin preparations". Proceedings of the National Academy of Sciences of the United States of America 74 (12): 5377–81. Dec 1977. doi:10.1073/pnas.74.12.5377. PMID 146197. Bibcode: 1977PNAS...74.5377K.
- "Interaction of synthetic human big gastrin with blood proteins of man and animals". Acta Hepato-Gastroenterologica 26 (2): 154–9. Apr 1979. PMID 463490.
- "Caeruloplasmin biosynthesis by the human uterus". The Biochemical Journal 288 (2): 657–61. Dec 1992. doi:10.1042/bj2880657. PMID 1463466.
- "Characterization of an interaction between protein C and ceruloplasmin". The Journal of Biological Chemistry 265 (4): 1834–6. Feb 1990. doi:10.1016/S0021-9258(19)39903-X. PMID 2105310.
- "Primary structure of rat ceruloplasmin and analysis of tissue-specific gene expression during development". The Journal of Biological Chemistry 265 (13): 7701–7. May 1990. doi:10.1016/S0021-9258(19)39171-9. PMID 2332446.
- "Human ceruloplasmin. Tissue-specific expression of transcripts produced by alternative splicing". The Journal of Biological Chemistry 265 (18): 10780–5. Jun 1990. doi:10.1016/S0021-9258(18)87015-6. PMID 2355023.
- "Characterization, mapping, and expression of the human ceruloplasmin gene". Proceedings of the National Academy of Sciences of the United States of America 83 (10): 3257–61. May 1986. doi:10.1073/pnas.83.10.3257. PMID 3486416. Bibcode: 1986PNAS...83.3257Y.
- "Isolation of a human ceruloplasmin cDNA clone that includes the N-terminal leader sequence". FEBS Letters 203 (2): 185–90. Jul 1986. doi:10.1016/0014-5793(86)80739-6. PMID 3755405.
- "Subcellular localization in normal and vitamin A-deficient rat liver of vitamin A serum transport proteins, albumin, ceruloplasmin and class I major histocompatibility antigens". Experimental Cell Research 143 (1): 91–102. Jan 1983. doi:10.1016/0014-4827(83)90112-X. PMID 6337857.
- "Origins of biliary copper". Hepatology 4 (5): 867–70. 1984. doi:10.1002/hep.1840040512. PMID 6479854.
- "Internal triplication in the structure of human ceruloplasmin". Proceedings of the National Academy of Sciences of the United States of America 80 (1): 115–9. Jan 1983. doi:10.1073/pnas.80.1.115. PMID 6571985. Bibcode: 1983PNAS...80..115T.
- "Complete amino acid sequence of a 50,000-dalton fragment of human ceruloplasmin". Proceedings of the National Academy of Sciences of the United States of America 78 (2): 790–4. Feb 1981. doi:10.1073/pnas.78.2.790. PMID 6940148. Bibcode: 1981PNAS...78..790D.
- "Primary structure of a histidine-rich proteolytic fragment of human ceruloplasmin. I. Amino acid sequence of the cyanogen bromide peptides". The Journal of Biological Chemistry 255 (7): 2878–85. Apr 1980. doi:10.1016/S0021-9258(19)85822-2. PMID 6987229.
External links
- GeneReviews/NCBI/NIH/UW entry on Aceruloplasminemia
- OMIM entries on Aceruloplasminemia
- Overview of all the structural information available in the PDB for UniProt: P00450 (Human Ceruloplasmin) at the PDBe-KB.
 |