Chemistry:Fibrin glue
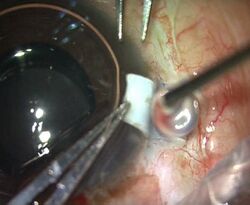 Fibrin glue applied after drying the scleral bed in an intraocular lens operation | |
| Combination of | |
|---|---|
| Fibrinogen | Glycoprotein |
| Thrombin | Coagulation factor |
| Clinical data | |
| Trade names | Artiss, Evicel, Tisseel, others |
| AHFS/Drugs.com | Professional Drug Facts |
| License data |
|
| Pregnancy category | |
| Routes of administration | Topical |
| ATC code | |
| Legal status | |
| Legal status | |
| Identifiers | |
| KEGG | |
Fibrin glue (also called fibrin sealant) is a surgical formulation used to create a fibrin clot for hemostasis, cartilage repair surgeries or wound healing. It contains separately packaged human fibrinogen and human thrombin.[15][16][17][18][19][20][21][22][23][24]
Medical uses
This glue is used as a supportive treatment in surgery (such as liver surgery) for the improvement of hemostasis where standard surgical techniques are insufficient or impractical.[13][8]
It is also used for repairing dura mater tears and bronchial fistulas and for achieving hemostasis after spleen and liver trauma,[23] in "no sutures" corneal transplantation, pterygium excision with amniotic membrane or conjunctival autograft, and in eye trauma for corneal or conjunctival defects,[25][26][27] as well as for skin graft donor site wounds to reduce postoperative pain.[28]
It can also be used to treat pilonidal sinus disease but it is of unclear benefit as of 2017, due to insufficient research.[29]
Contraindications
The glue must not get into blood vessels, as this could lead to clotting in the form of thromboembolism or disseminated intravascular coagulation, or to anaphylaxis (a severe allergic reaction).[30]
Side effects
Possible adverse effects include bleeding disorder and allergic reactions such as flushing, stinging, generalised urticaria, angioedema, bronchospasm, and anaphylaxis. Other adverse effects in studies occurred in roughly equal proportions in treatment and placebo groups.[30]
Interactions
As fibrin glue contains proteins, it may be denatured by ethanol, iodine and heavy metals. These substances are frequently found in antiseptic solutions.[30]
Pharmacology
Mechanism of action
Thrombin is an enzyme that splits fibrinogen into fibrin monomers in 10 to 60 seconds, which aggregate to form a three-dimensional gel-like structure. Thrombin also activates factor XIII from the human body to factor XIIIa, which then cross-links the fibrin monomers to form a stable clot. Both these processes need calcium to work. As the wound heals, the clot is slowly degraded by the enzyme plasmin.[23][30][31]
Pharmacokinetics
In rabbit studies, only 1 to 2% of the applied thrombin dose reached the bloodstream. It reached highest blood plasma concentrations after 6 to 8 hours.[30]
Chemistry
Composition
Fibrin glue comes in two vials, respectively containing:
- fibrinogen: lyophilised pooled human concentrate
- thrombin: This used to be of bovine origin; modern formulations contain human thrombin.[18]
The two components are mixed immediately before application.[13][32] The formulations also contain calcium salts.[30]
Formulations from different manufacturers may also contain aprotinin, fibronectin, plasminogen, and factor XIII.[33][34]
Legal status
A formulation with human thrombin was approved for medical use in the United States in March 2003, and in the European Union in October 2008.[13][32][35]
References
- ↑ 1.0 1.1 "VeraSeal". 12 November 2021. https://www.tga.gov.au/apm-summary/veraseal.
- ↑ "Updates to the Prescribing Medicines in Pregnancy database". 12 May 2022. https://www.tga.gov.au/resources/resource/guidance/updates-prescribing-medicines-pregnancy-database.
- ↑ "AusPAR: Human Fibrinogen / Human Thrombin". 29 June 2022. https://www.tga.gov.au/auspar/auspar-human-fibrinogen-human-thrombin-0.
- ↑ "Health product highlights 2021: Annexes of products approved in 2021". 3 August 2022. https://www.canada.ca/en/health-canada/services/publications/drugs-health-products/health-product-highlights-2021/appendices.html.
- ↑ "TachoSil sealant matrix - Summary of Product Characteristics (SmPC)". 31 January 2020. https://www.medicines.org.uk/emc/product/119/smpc.
- ↑ "Tisseel Ready to use Solutions for Sealant - Summary of Product Characteristics (SmPC)". 30 December 2019. https://www.medicines.org.uk/emc/product/1801/smpc.
- ↑ "Artiss Solutions for Sealant - Summary of Product Characteristics (SmPC)". 29 January 2019. https://www.medicines.org.uk/emc/product/1800/smpc.
- ↑ 8.0 8.1 "Evicel Fibrin Sealant (Human)". 22 July 2017. https://www.fda.gov/vaccines-blood-biologics/approved-blood-products/evicel.
- ↑ "Vistaseal". 1 October 2024. https://www.fda.gov/vaccines-blood-biologics/approved-blood-products/vistaseal.
- ↑ "Artiss Fibrin Sealant- fibrinogen human, human thrombin solution". 9 June 2022. https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=92fc93e1-9695-4705-90c5-4452a80bb074.
- ↑ "Tisseel Fibrin Sealant- fibrinogen human, human thrombin kit; Tisseel Fibrin Sealant- fibrinogen human, human thrombin solution". 26 December 2019. https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=b3f69e25-1b87-4507-81fa-582ea084673d.
- ↑ "TachoSil". 1 October 2024. https://www.fda.gov/vaccines-blood-biologics/approved-blood-products/tachosil.
- ↑ 13.0 13.1 13.2 13.3 "Evicel EPAR". 17 September 2018. https://www.ema.europa.eu/en/medicines/human/EPAR/evicel.
 This article incorporates text from this source, which is in the public domain.
This article incorporates text from this source, which is in the public domain.
- ↑ "Veraseal EPAR". 10 November 2017. https://www.ema.europa.eu/en/medicines/human/EPAR/veraseal-0.
- ↑ "The clot thickens: Autologous and allogeneic fibrin sealants are mechanically equivalent in an ex vivo model of cartilage repair". PLOS ONE 14 (11). 8 November 2019. doi:10.1371/journal.pone.0224756. PMID 31703078. Bibcode: 2019PLoSO..1424756I.
- ↑ "Current state and use of biological adhesives in orthopedic surgery". Orthopedics 36 (12): 945–956. December 2013. doi:10.3928/01477447-20131120-09. PMID 24579215.
- ↑ "Autologous Fibrin Sealants Have Comparable Graft Fixation to an Allogeneic Sealant in a Biomechanical Cadaveric Model of Chondral Defect Repair" (in English). Arthroscopy, Sports Medicine, and Rehabilitation 4 (3): e1075–e1082. 15 April 2022. doi:10.1016/j.asmr.2022.03.003. ISSN 2666-061X. PMID 35747626.
- ↑ 18.0 18.1 "Fibrin glue". BMJ 308 (6934): 933–934. April 1994. doi:10.1136/bmj.308.6934.933. PMID 8173397.
- ↑ "Fibrin glue: a review of its preparation, efficacy, and adverse effects as a topical hemostat". Drug Intelligence & Clinical Pharmacy 22 (12): 946–952. December 1988. doi:10.1177/106002808802201203. PMID 2468466.
- ↑ "Fibrin Glue for Anal Fistula - Digestive Disorders / Gastroenterology". MedHelp. http://www.medhelp.org/forums/gastro/archive/1054.html.
- ↑ "Effect of fibrin glue on small and large bowel anastomoses in the rat". European Surgical Research 30 (1): 8–12. 1998. doi:10.1159/000008552. PMID 9493689.
- ↑ "Fibrin glue from stored human plasma. An inexpensive and efficient method for local blood bank preparation". The American Surgeon 53 (8): 460–462. August 1987. PMID 2440358.
- ↑ 23.0 23.1 23.2 "Preparation of two component Fibrin Glue and its clinical evaluation in skin grafts and flaps". Indian J Plast Surg 36 (1): 14–17. 2003. doi:10.1055/s-0043-1778572. http://www.ijps.org/article.asp?issn=0970-0358;year=2003;volume=36;issue=1;spage=14;epage=17;aulast=Saxena.
- ↑ "Performing microvascular anastomosis with fibrin glue--faster, easier, and more reliable?". Microsurgery 29 (1): 80–81. 2009. doi:10.1002/micr.20556. PMID 18946885.
- ↑ "No sutures corneal grafting--a novel use of overlay sutures and fibrin glue in Deep Anterior Lamellar Keratoplasty". Contact Lens & Anterior Eye 30 (3): 207–209. July 2007. doi:10.1016/j.clae.2007.02.007. PMID 17379570.
- ↑ "[Use of fibrin glue in ocular surgery]" (in pt). Arquivos Brasileiros de Oftalmologia 72 (3): 308–312. 2009. doi:10.1590/s0004-27492009000300006. PMID 19668958.
- ↑ "Fibrin Sealant Fibrin Gluing Haemostasis autologous". vivostat.com. http://www.vivostat.com/products/vivostat-fibrin-sealant.
- ↑ "Treating pain on skin graft donor sites: Review and clinical recommendations". The Journal of Trauma and Acute Care Surgery 83 (5): 954–964. November 2017. doi:10.1097/TA.0000000000001615. PMID 28598907.
- ↑ "Fibrin glue for pilonidal sinus disease". The Cochrane Database of Systematic Reviews 1 (1). January 2017. doi:10.1002/14651858.CD011923.pub2. PMID 28085995.
- ↑ 30.0 30.1 30.2 30.3 30.4 30.5 "Evicel: EPAR – Product Information". European Medicines Agency. 12 June 2020. https://www.ema.europa.eu/en/documents/product-information/evicel-epar-product-information_en.pdf.
- ↑ "Fibrin sealant: past, present, and future: a brief review". World Journal of Surgery 34 (4): 632–634. April 2010. doi:10.1007/s00268-009-0252-7. PMID 19820991.
- ↑ 32.0 32.1 "Evicel Fibrin Sealant (Human)- fibrinogen human and thrombin human kit". 17 December 2019. https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=31dc7ff6-bdd8-4e65-897b-109b6f8316b6.
- ↑ "Tisseel". Swedish official drug catalog. http://www.fass.se/LIF/produktfakta/artikel_produkt.jsp?NplID=19950119000010&DocTypeID=5.
- ↑ KEGG drug: Factor XIII with fibrinogen. Accessed 9 July 2020.
- ↑ "Evicel". 5 June 2017. https://www.fda.gov/BiologicsBloodVaccines/BloodBloodProducts/ApprovedProducts/LicensedProductsBLAs/FractionatedPlasmaProducts/ucm089269.htm.
 |
