Biology:Insect cognition
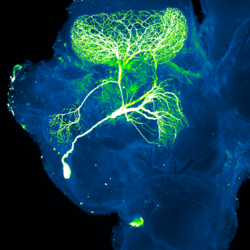
Insect cognition describes the mental capacities and study of those capacities in insects. The field developed from comparative psychology where early studies focused more on animal behavior.[1] Researchers have examined insect cognition in bees, fruit flies, and wasps.[2][3]
Research questions consist of experiments aimed to evaluate insects abilities such as perception,[4] emotions[1][5] attention,[3] memory (wasp multiple nest),[1] spatial cognition,[1][6] tools use,[3] problem solving,[3] and concepts.[3][7] Unlike in animal behavior the concept of group cognition plays a big part in insect studies.[7][8][9] It is hypothesized some insect classes like ants and bees think with a group cognition to function within their societies;[8][9] more recent studies show that individual cognition exists and plays a role in overall group cognitive task.[5]
Insect cognition experiments have been more prevalent in the past decade than prior.[3] It is logical for the understanding of cognitive capacities as adaptations to differing ecological niches under the Cognitive faculty by species when analyzing behaviors, this means viewing behaviors as adaptations to an individual's environment and not weighing them more advanced when compared to other different individuals.[10]
Insect foraging cognition

Insects inhabit many diverse and complex environments within which they must find food. Cognition shapes how an insect comes to find its food. The particular cognitive abilities used by insects in finding food has been the focus of much scientific inquiry.[11] The social insects are often study subjects and much has been discovered about the intelligence of insects by investigating the abilities of bee species.[12][3] Fruit flies are also common study subjects.[13]
Learning and memory
Learning biases
Through learning, insects can increase their foraging efficiency, decreasing the time spent searching for food which allows for more time and energy to invest in other fitness related activities, such as searching for mates. Depending on the ecology of the insect certain cues may be used to learn to quickly identify food sources. Over evolutionary time insects may develop evolved learning biases that reflect the food source they feed on.[14]
Biases in learning allow insects to quickly associate relevant features of the environment that are related to food. For example, bees have an unlearned preference for radiating and symmetric patterns — features of natural flowers bees forage on.[15] Bees that have no foraging experience tend to have an unlearned preference for the colours that an experienced forager would learn faster. These colours tend to be those of highly rewarding flowers in that particular environment.[16]
Time-place learning
In addition to more typical cues like color and odor, insects are able to use time as a foraging cue.[17] Time is a particularly important cue for pollinators. Pollinators forage on flowers which tend to vary predictably in time and space, depending on the flower species, pollinators can learn the timing of blooming of flower species to develop more efficient foraging routes. Bees learn at which times and in which areas sites are rewarding and change their preference for particular sites based on the time of day.[18]
These time-based preferences have been shown to be tied to a circadian clock in some insects. In the absence of external cues honeybees will still show a shift in preference for a reward depending on time strongly implicating an internal time-keeping mechanism, i.e. the circadian clock, in modulating the learned preference.[17]
Moreover, not only can bees remember when a particular site is rewarding but they can also remember at what times multiple different sites are profitable.[18] Certain butterfly species also show evidence for time-place learning due to their trap-line foraging behaviour.[19] This is when an animal consistently visits the same foraging sites in a sequential manner across multiple days and is thought to be suggestive of a time-place learning ability.
Innovation capacity
File:Associative-Mechanisms-Allow-for-Social-Learning-and-Cultural-Transmission-of-String-Pulling-in-an-pbio.1002564.s006.ogv Insects are also capable of behavioral innovations. Innovation is defined as the creation of a new or modified learned behavior not previously found in the population.[21] Innovative abilities can be experimentally studied in insects through the use of problem solving tasks.[22] When presented with a string-pulling task, many bumblebees cannot solve the task, but a few can innovate the solution.[20]
Those that initially could not solve the task can learn to solve it by observing an innovator bee solving the task. These learned behaviors can then spread culturally through bee populations.[20] More recent studies in insects have begun to look at what traits (e.g. exploratory tendency) predict the propensity for an individual insect to be an innovator.[23]
Social aspects of insect foraging
Social learning of foraging sites
Insects can learn about foraging sites through observation or interaction with other individuals, termed social learning. This has been demonstrated in bumblebees. Bumblebees become attracted to rewarding flowers more quickly if they are occupied by other bumblebees and more quickly learn to associate that flower species with reward.[24] Seeing a conspecific on a flower enhances preferences for flowers of that type. Additionally, bumblebees will rely more on social cues when a task is difficult compared to when a task is simple.[25]
Ants will show conspecifics food sites they have discovered in a process called tandem running. This is considered to be a rare instance of teaching, a specialized form of social learning, in the animal kingdom.[26] Teaching involves consistent interactions between a tutor and a pupil and the tutor typically incurs some sort of cost in order to transmit the relevant information to the pupil. In the case of tandem running the ant is temporarily decreasing its own foraging efficiency in order to demonstrate to the pupil the location of a foraging site.
Evidence for Cumulative culture
Studies in bumblebees have provided evidence that some insects show the beginnings of cumulative culture through the act of refining existing behaviours into more efficient forms. Bumblebees are able to improve upon a task where they must pull a ball to a particular location, a previously socially learned behaviour, by using a more optimal route compared to the route that their demonstrator used.[27] This demonstration of refinement of a previously observed existing behaviour could be considered a rudimentary form of cumulative culture, although this a highly controversial idea. It is important to say that true cumulative culture has been difficult to show in insects and indeed, in all species. This would require culture accumulating over generations to the point where no single individual could independently generate the entire behaviour.[28]
Neural basis of insect foraging
Role of mushroom bodies
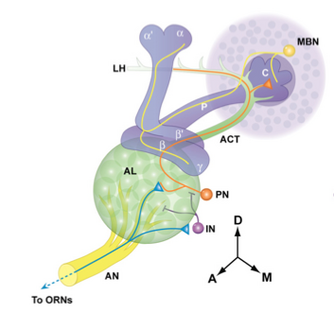
One important and highly studied brain region involved in insect foraging are the mushroom bodies, a structure implicated in insect learning and memory abilities. The mushroom body consists of two large stalks called peduncles which have cup-shaped projections on their ends called calyces. The role of the mushroom bodies is in sensory integration and associative learning.[29] They allow the insect to pair sensory information and reward.[29]
Experiments where the function of the mushroom bodies are impaired through ablation find that organisms are behaviourally normal but have impaired learning. Flies with impaired mushroom bodies cannot form an odour association[30] and cockroaches with impaired mushroom bodies cannot make use of spatial information to form memories about locations.[31] Electrophysiological underpinnings of the cognition in different parts of the insect brain can be studied by various techniques including in vivo recordings from these parts of the insect brain.
Mushroom body plasticity
Mushroom bodies can change in size throughout the lifespan of an insect. There is evidence these changes are related to the onset of foraging as well as the experience of foraging. In some Hymenoptera mushroom bodies increase in size when nurses become foragers and begin to forage for the colony.[32]
Young bees begin as nurses tending to the feeding and sanitation of the hive's larvae. As a bee ages it undergoes a shift in tasks from nurse to forager, leaving the hive to collect pollen. This shift in job leads to changes in gene expression within the brain which are associated with an increase in mushroom body size.[32]
Some butterflies have also been shown to undergo an experience-dependent increase in mushroom body size.[33] The period of greatest increase in brain size typically is associated with a period of learning through experiences with foraging demonstrating the importance of this structure in insect foraging cognition.
Mushroom body evolution
Multiple insect taxa have independently evolved larger mushroom bodies. The spatial cognition demands of foraging has been implicated in cases where more sophisticated mushroom bodies have evolved.[34] Cockroaches and bees, which are in different orders, both forage over a large area and make use of spatial information to return to foraging sites and central places which likely explains their larger mushroom bodies.[35] Contrast this with a dipteran such as the fruit fly Drosophila melanogaster, which has relatively small mushroom bodies and less complex spatial learning demands.
References
- ↑ 1.0 1.1 1.2 1.3 Burkhardt, Richard W. (1987). "The Journal of Animal Behavior and the early history of animal behavior studies in America.". Journal of Comparative Psychology 101 (3): 223–230. doi:10.1037/0735-7036.101.3.223. ISSN 0735-7036.
- ↑ "Cognition with few neurons: higher-order learning in insects". Trends in Neurosciences 36 (5): 1 285–294. 2014. doi:10.1016/j.tins.2012.12.011. PMID 23375772.
- ↑ 3.0 3.1 3.2 3.3 3.4 3.5 3.6 "The frontiers of insect cognition". Current Opinion in Behavioral Sciences 16: 111–118. 2017. doi:10.1016/j.cobeha.2017.05.011. ISSN 2352-1546.
- ↑ "Insect visual perception: complex abilities of simple nervous systems". Current Opinion in Neurobiology 7 (4): 505–513. 1997. doi:10.1016/S0959-4388(97)80030-X. PMID 9287201.
- ↑ 5.0 5.1 "Do Insects Have Emotions? Some Insights from Bumble Bees". Frontiers in Behavioral Neuroscience 11: 157. August 2017. doi:10.3389/fnbeh.2017.00157. PMID 28878636.
- ↑ "Blackawton bees". Biology Letters 7 (2): 168–72. April 2011. doi:10.1098/rsbl.2010.1056. PMID 21177694.
- ↑ 7.0 7.1 Passino, Kevin M.; Seeley, Thomas D.; Visscher, P. Kirk (2007-09-19). "Swarm cognition in honey bees". Behavioral Ecology and Sociobiology 62 (3): 401–414. doi:10.1007/s00265-007-0468-1. ISSN 0340-5443.
- ↑ 8.0 8.1 Wilson, Robert A. (September 2001). "Group-Level Cognition". Philosophy of Science 68 (S3): S262–S273. doi:10.1086/392914. ISSN 0031-8248.
- ↑ 9.0 9.1 "Individual versus collective cognition in social insects". The Journal of Experimental Biology 220 (Pt 1): 73–82. January 2017. doi:10.1242/jeb.143891. PMID 28057830. Bibcode: 2017arXiv170105080F.
- ↑ "Cognition with few neurons: higher-order learning in insects". Trends in Neurosciences 36 (5): 285–94. May 2013. doi:10.1016/j.tins.2012.12.011. PMID 23375772.
- ↑ "Learning in Insect Pollinators and Herbivores". Annual Review of Entomology 62 (1): 53–71. January 2017. doi:10.1146/annurev-ento-031616-034903. PMID 27813668.
- ↑ Bees Have Small Brains But Big Ideas. doi:10.1038/scientificamericanmind0514-10b. https://www.scientificamerican.com/article/bees-have-small-brains-but-big-ideas/.
- ↑ Maimon, Gaby. "Could Fruit Flies Reveal the Hidden Mechanisms of the Mind?" (in en). https://blogs.scientificamerican.com/observations/could-fruit-flies-reveal-the-hidden-mechanisms-of-the-mind/.
- ↑ "Shape vision in bees: innate preference for flower-like patterns" (in en). Philosophical Transactions of the Royal Society of London. Series B: Biological Sciences 347 (1320): 123–137. 1995-01-30. doi:10.1098/rstb.1995.0017. ISSN 0962-8436. Bibcode: 1995RSPTB.347..123L. http://eprints.iisc.ac.in/67757/1/Lehrer%20et%20al%201995-Phil%20Trans%20R%20Soc%20Lond%20B.pdf.
- ↑ "Shape vision in bees: innate preference for flower-like patterns" (in en). Philosophical Transactions of the Royal Society of London. Series B: Biological Sciences 347 (1320): 123–137. 1995-01-30. doi:10.1098/rstb.1995.0017. ISSN 0962-8436. Bibcode: 1995RSPTB.347..123L. http://eprints.iisc.ac.in/67757/1/Lehrer%20et%20al%201995-Phil%20Trans%20R%20Soc%20Lond%20B.pdf.
- ↑ "Colour preferences of flower-naive honeybees" (in en). Journal of Comparative Physiology A 177 (3). September 1995. doi:10.1007/BF00192415. ISSN 0340-7594.
- ↑ 17.0 17.1 "Honeybee memory: A honeybee knows what to do and when". The Journal of Experimental Biology 209 (Pt 22): 4420–8. November 2006. doi:10.1242/jeb.02522. PMID 17079712.
- ↑ 18.0 18.1 "Circadian timed episodic-like memory - a bee knows what to do when, and also where". The Journal of Experimental Biology 210 (Pt 20): 3559–67. October 2007. doi:10.1242/jeb.005488. PMID 17921157.
- ↑ Gilbert, Lawrence E. Raven, Peter H. (1980). Coevolution of animals and plants : Symposium V, First International Congress of Systematic and Evolutionary Biology, Boulder, Colorado, August 1973. University of Texas. ISBN 0-292-71056-9. OCLC 6511746.
- ↑ 20.0 20.1 20.2 "Associative Mechanisms Allow for Social Learning and Cultural Transmission of String Pulling in an Insect". PLOS Biology 14 (10): e1002564. October 2016. doi:10.1371/journal.pbio.1002564. PMID 27701411.
- ↑ Reader, Simon M.; Laland, Kevin N. (2003-09-25). "Animal Innovation: An Introduction". Animal Innovation. Oxford University Press. pp. 3–36. doi:10.1093/acprof:oso/9780198526223.003.0001. ISBN 978-0-19-852622-3.
- ↑ "Innovation and problem solving: a review of common mechanisms". Behavioural Processes 109 Pt B: 121–34. November 2014. doi:10.1016/j.beproc.2014.08.027. PMID 25245306.
- ↑ Collado, Miguel Á.; Menzel, Randolf; Sol, Daniel; Bartomeus, Ignasi (2019-12-23) (in en). Innovation in solitary bees is driven by exploration, shyness and activity levels. doi:10.1101/2019.12.23.884619.
- ↑ Leadbeater, Ellouise; Chittka, Lars (2007-08-06). "The dynamics of social learning in an insect model, the bumblebee (Bombus terrestris)" (in en). Behavioral Ecology and Sociobiology 61 (11): 1789–1796. doi:10.1007/s00265-007-0412-4. ISSN 0340-5443.
- ↑ Baracchi, David; Vasas, Vera; Jamshed Iqbal, Soha; Alem, Sylvain (2018-01-13). Papaj, Dan. ed. "Foraging bumblebees use social cues more when the task is difficult" (in en). Behavioral Ecology 29 (1): 186–192. doi:10.1093/beheco/arx143. ISSN 1045-2249.
- ↑ "Teaching in tandem-running ants". Nature 439 (7073): 153. January 2006. doi:10.1038/439153a. PMID 16407943. Bibcode: 2006Natur.439..153F.
- ↑ "Bumblebees show cognitive flexibility by improving on an observed complex behavior". Science 355 (6327): 833–836. February 2017. doi:10.1126/science.aag2360. PMID 28232576. Bibcode: 2017Sci...355..833L. https://zenodo.org/record/889455.
- ↑ "Cumulative culture in nonhumans: overlooked findings from Japanese monkeys?". Primates 59 (1): 113–122. December 2017. doi:10.1007/s10329-017-0642-7. PMID 29282581. Bibcode: 2017Sci...355..833L.
- ↑ 29.0 29.1 "Mushroom body memoir: from maps to models". Nature Reviews. Neuroscience 4 (4): 266–75. April 2003. doi:10.1038/nrn1074. PMID 12671643.
- ↑ "Associative odor learning in Drosophila abolished by chemical ablation of mushroom bodies". Science 263 (5147): 692–5. February 1994. doi:10.1126/science.8303280. PMID 8303280. Bibcode: 1994Sci...263..692D.
- ↑ Mizunami, Makoto; Weibrecht, Josette M.; Strausfeld, Nicholas J. (1998-12-28). "Mushroom bodies of the cockroach: Their participation in place memory". The Journal of Comparative Neurology 402 (4): 520–537. doi:10.1002/(sici)1096-9861(19981228)402:4<520::aid-cne6>3.0.co;2-k. ISSN 0021-9967. PMID 9862324.
- ↑ 32.0 32.1 "Gene expression profiles in the brain predict behavior in individual honey bees". Science 302 (5643): 296–9. October 2003. doi:10.1126/science.1086807. PMID 14551438. Bibcode: 2003Sci...302..296W.
- ↑ "Brain composition in Heliconius butterflies, posteclosion growth and experience-dependent neuropil plasticity". The Journal of Comparative Neurology 524 (9): 1747–69. June 2016. doi:10.1002/cne.23993. PMID 26918905. https://figshare.com/articles/journal_contribution/10173215.
- ↑ "Parasitoidism, not sociality, is associated with the evolution of elaborate mushroom bodies in the brains of hymenopteran insects". Proceedings. Biological Sciences 278 (1707): 940–51. March 2011. doi:10.1098/rspb.2010.2161. PMID 21068044.
- ↑ Farris, Sarah M; Van Dyke, Joseph W (December 2015). "Evolution and function of the insect mushroom bodies: contributions from comparative and model systems studies" (in en). Current Opinion in Insect Science 12: 19–25. doi:10.1016/j.cois.2015.08.006.
Further reading
- Brebner, Joanna; Chittka, Lars (2021-02-22). "Animal Cognition: The Self-Image of a Bumblebee" (in en). Current Biology 31 (4): R207–R209. doi:10.1016/j.cub.2020.12.027. ISSN 0960-9822. PMID 33621512.
 |
