Biology:Argentine ant
| Argentine ant | |
|---|---|

| |
| Scientific classification Error creating thumbnail: Unable to save thumbnail to destination
| |
| Domain: | Eukaryota |
| Kingdom: | Animalia |
| Phylum: | Arthropoda |
| Class: | Insecta |
| Order: | Hymenoptera |
| Family: | Formicidae |
| Subfamily: | Dolichoderinae |
| Genus: | Linepithema |
| Species: | L. humile
|
| Binomial name | |
| Linepithema humile (Mayr, 1868)
| |
The Argentine ant (Linepithema humile, formerly Iridomyrmex humilis) is an ant native to northern Argentina , Uruguay, Paraguay, Bolivia and southern Brazil .[1] This invasive species was inadvertently introduced by humans on a global scale[2][3][4] and has become established in many Mediterranean climate areas,[5][6][7][8] including South Africa ,[9] New Zealand,[10] Japan ,[11] Easter Island,[12] Australia ,[13] Europe,[14] Hawaii,[15] and the continental United States.[16] Argentine ants are significant pests within agricultural and urban settings,[17][18][19] and are documented to cause substantial harm to communities of native arthropods,[20][21][22] vertebrates,[23][24][25] and plants[26][27][28] within their invaded range.
Description
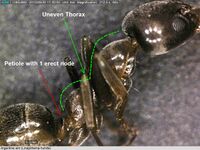
Linepithema humile is a small-bodied (2.2–2.6 mm) ant species, dull light to dark brown in color. Within the invasion zone, ant colonies are large and include many workers and multiple queens.[29]
Argentine ants are opportunistic with regard to nesting preferences. Colony nests have been found in the ground, in cracks in concrete walls, in spaces between boards and timbers, even among belongings in human dwellings. In natural areas, they generally nest shallowly in loose leaf litter or beneath small stones, due to their poor ability to dig deeper nests.[30] However, if a deeper nesting ant species abandons their nest, Argentine ant colonies will readily take over the space.[citation needed] Because the native habitat for this species is within riparian floodplains, colonies are very sensitive to water infiltration within their nests; if their nests become inundated with water, workers will collect the brood and the entire colony will move to dry ground.[31][32]
Austrian entomologist Gustav L. Mayr identified the first specimens of Hypoclinea humilis in the vicinity of Buenos Aires, Argentina in 1866. This species was shortly transferred to the genus Iridomyrmex, and finally to Linepithema in the early 1990s.
Distribution
The native range of Argentine ants is limited to riparian habitats in the lowland areas of the Paraná River drainage,[5][8] which stretches across northern Argentina, Uruguay, Paraguay, and southern Brazil. Within South America, this species has spread into parts of Chile , Colombia, Ecuador, and Peru.[33] Linepithema humile thrives in Mediterranean climates, and over the past century it has spread to across the globe by human-mediated transport.[5] The species has become established to every continent except Antarctica and includes many oceanic islands. [2][34][35]
Global "mega-colony"
The absence of aggression within Argentine ant colonies was first reported in 1913 by Newell & Barber, who noted "…there is no apparent antagonism between separate colonies of its own kind".[36] Later studies showed that these "supercolonies" extend across hundreds or thousands of kilometers in different parts of the introduced range, first reported in California in 2000,[34] then in Europe in 2002,[37] Japan in 2009,[38]:{{{1}}} and Australia in 2010.[39] Several subsequent studies used genetic, behavioral, and chemical analyses to show that introduced supercolonies on separate continents actually represent a single global supercolony.[40][38]:{{{1}}}
The researchers stated that the "enormous extent of this population is paralleled only by human society", and had probably been spread and maintained by human travel.[38]:{{{1}}}
Behavior

They have been extraordinarily successful, in part, because different nests of the introduced Argentine ants seldom attack or compete with each other, unlike most other species of ant. In their introduced range, their genetic makeup is so uniform that individuals from one nest can mingle in a neighboring nest without being attacked. Thus, in most of their introduced range, they form supercolonies.[41] The Very Large Colony, which covers territory from San Diego to beyond San Francisco , may have a population of nearly one trillion individuals.[42]:{{{1}}}
Conflict does occur between members of different supercolonies. In 1997, UC San Diego researchers observed fighting between different Argentine ants kept in lab, and in 2004 scientists began to map out the boundaries of the different supercolonies that clashed in San Diego. On the border of the Very Large Colony and the Lake Hodges Colony thirty million ants die each year, on a battlefront that covers many miles. While the battles of other ant species generally constitute colony raids lasting a few hours, or skirmishes that occur periodically for a few weeks, Argentine ants clash ceaselessly; the borders of their territory are a site of constant violence and battles can be fought on top of hundreds of dead ants. Fights may be halted by adverse weather such as rain.[42]:{{{1}}}
In contrast, native populations are genetically more diverse and form colonies that are much smaller than the supercolonies that dominate the introduced range. Colonies living in close proximity are territorial and aggressive toward one another. Argentine ants in their native South America also co-exist with many other species of ants, and do not attain the high population densities that characterize introduced populations.[43]
In a series of experiments, ants of the same colony were isolated and fed different diets. The hydrocarbons from the diet were eventually incorporated into the cuticle of the subjects. Those that had the same diet appeared to recognize one another as kin. Those who had at least some overlap in dietary composition also appeared to react non-aggressively to one another. These interactions contrasts drastically with the groups that fed on completely different sources, such as those who lived off flies and those that fed on grasshoppers. The groups appeared to have incorporated hydrocarbons that were not similar to the others and created an unfamiliar identity cue. These groups reacted violently towards each other. This suggests that dietary factors affect the recognition cues for colony members.[44][45]
Reproduction and seasonal colony trends
Like workers in many other ant species, Argentine ant workers are unable to lay reproductive eggs but can direct the development of eggs into reproductive females; the production of males appears to be controlled by the amount of food available to the larvae.[46] Argentine ant colonies almost invariably have many reproductive queens, as many as eight for every 1,000 workers.
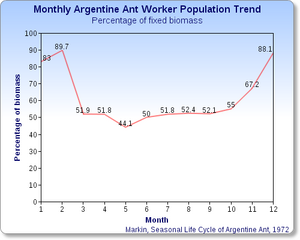
The seasonal low occurs in mid-winter, when 90% of a representative colony consists of workers and the remainder of queens, and no reproductive activity and minimal birthing. Eggs are produced in late-winter, nearly all of which hatch into sexual forms by May(*). Mating occurs after the females emerge. Worker production increases steadily from mid-March(*) to October(*), after which their numbers are not replenished; thus, their numbers drop steadily over the winter months.[30] ((*) Note that the information regarding the months May, March, and October in this paragraph, as well as the entire Month axis in the graph, are most likely not worldwide correct, in special in Southern hemisphere, due to the shift of six months in seasons between the two hemispheres.)
Colonies in the Argentine ant's native habitat are kept within a range of ten to one hundred meters by colonies of interspecific and intraspecific rivals. As the colonies expand, they appear to form fluctuating territory borders, which contract and expand on a seasonal and conditional basis. There is an expansive push outward in the summer months, with a retreating motion in the winter. This has to do with soil moisture and temperature conditions.[47] At the edges of these borders are either rival L. humile colonies or other obstacles that prevent further expansion, such as an inhospitable environment for nests.[47]
Impact
The ants are ranked among the world's 100 worst invasive animal species.[48] In its introduced range, the Argentine ant often displaces most or all native ants and can threaten native invertebrates and even small vertebrates that are not accustomed to defending against the aggressive ants. This can, in turn, imperil other species in the ecosystem, such as native plants that depend on native ants for seed dispersal, or lizards that depend on native ants or invertebrates for food. For example, the recent severe decline in coastal horned lizards in southern California is closely tied to Argentine ants displacing native ant species on which the lizards feed.[25]
Argentine ants sometimes tend aphid, mealybug, and scale insect colonies,[49] sometimes relocating the parasites to unaffected plants, and their protection of these plant pests from predators and parasitoids can cause problems in agricultural areas.[50] In return for this protection, the ants benefit by feeding off an excretion known as "honeydew". Thus, when Argentine ants invade an agricultural area, the population densities of these plant parasites can increase followed by an increase in damage to crops.[citation needed]
There is also evidence that the presence of Argentine ant may decrease the number of pollinators that visit natural flowering plants via predation on the larvae of the pollinators.[51]
Pest control
Argentine ants are a common household pest, often entering structures in search of food or water (particularly during dry or hot weather), or to escape flooded nests during periods of heavy rainfall. When they invade a kitchen, it is not uncommon to see two or three queens foraging along with the workers. Due to the large number of queens, eliminating a single queen does not stop the colony's ability to breed.[citation needed]
Diatomaceous earth has been used to dust trails, feeding sites and nest entrances.[citation needed]
Borate-sucrose water baits are toxic to Argentine ants, when the bait is 25% water, with 0.5–1.0% boric acid or borate salts.[52][53]
In spring, during a colony's growth phase, protein based baits may be more effective due to much higher demand from the egg-laying queens.[54]
Due to their nesting behavior and presence of numerous queens in each colony, it is generally impractical to spray Argentine ants with pesticides or to use boiling water as with mound building ants. Spraying with pesticides has occasionally stimulated increased egg-laying by the queens, compounding the problem. Pest control usually requires exploiting their omnivorous dietary habits, through use of slow-acting poison bait (e.g. fipronil, hydramethylnon, sulfluramid), which will be carried back to the nest by the workers, eventually killing all the individuals, including the queens. It may take four to five days to eradicate a colony in this manner.[citation needed]
Researchers from the University of California, Irvine, have developed a way to use the scent of Argentine ants against them.[55] The exoskeletons of the ants are covered with a hydrocarbon-laced secretion. They made a compound that is different, but similar, to the one that coats the ants. If the chemical is applied to an ant, the other members of the colony will kill it.[56] The chemical method may be effective in combination with other methods.[citation needed]
Another approach for a large scale control of the Argentine ant has been proposed by researchers from Japan, who showed that it is possible to disrupt its trails with synthetic pheromones.[57] This has been confirmed in various later trials by a New Zealand-led team in Hawaii[58] and by researchers from Victoria University of Wellington who showed that this approach is beneficial for other local ant species.[59]
See also
- Linepithema humile virus 1, a virus carried by Argentine ants
References
- ↑ Hölldobler, B.; Wilson, E. O. (1990). The Ants. Belknap Press. ISBN 9780674040755.
- ↑ 2.0 2.1 Wetterer, James K; Wild, Alexander L; Suarez, Andrew V; Roura-Pascual, Núria; Espadaler, Xavier (2009). "Worldwide spread of the Argentine ant, Linepithema humile (Hymenoptera: Formicidae)". Myrmecological News 12: 187–194. https://myrmecologicalnews.org/cms/index.php?option=com_content&view=category&id=377&Itemid=356.
- ↑ Vogel, Valérie; Pedersen, Jes S.; Giraud, Tatiana; Krieger, Michael J. B.; Keller, Laurent (2010). "The worldwide expansion of the Argentine ant". Diversity and Distributions 16 (1): 170–186. doi:10.1111/j.1472-4642.2009.00630.x. ISSN 1366-9516. http://dx.doi.org/10.1111/j.1472-4642.2009.00630.x.
- ↑ Wild, Alexander L. (2004). "Taxonomy and distribution of the Argentine ant, Linepithema humile (Hymenoptera: Formicidae)". Annals of the Entomological Society of America 97 (6): 1204–1215. doi:10.1603/0013-8746(2004)097[1204:TADOTA2.0.CO;2].
- ↑ 5.0 5.1 5.2 These reviews... Holway, David; Lach, Lori; Suarez, Andrew; Tsutsui, Neil; Case, Ted (2002). "The Causes and Consequences of Ant Invasions". Annual Review of Ecology and Systematics (Annual Reviews) 33 (1): 181–233. doi:10.1146/annurev.ecolsys.33.010802.150444. ISSN 0066-4162. https://www.life.illinois.edu/suarez/publications/Holway_etal2002ARES.pdf. Retrieved 28 February 2023. https://www.life.illinois.edu/suarez/publications/Holway_etal2002ARES.pdf (dead) Suarez, Andrew; Tsutsui, Neil (2008). "The evolutionary consequences of biological invasions". Molecular Ecology (Blackwell Science Ltd.) 17 (1): 351–360. doi:10.1111/j.1365-294x.2007.03456.x. ISSN 0962-1083. PMID 18173507. http://www.life.illinois.edu/suarez/publications/SuarezTsutsui2008MolEcol.pdf. Retrieved 28 February 2023. https://www.life.illinois.edu/suarez/publications/SuarezTsutsui2008MolEcol.pdf (dead) ...cite this study: Tsutsui, Neil; Suarez, Andrew; Holway, David; Case, Ted (2001). "Relationships among native and introduced populations of the Argentine ant (Linepithema humile) and the source of introduced populations". Molecular Ecology (Blackwell Science Ltd.) 10 (9): 2151–2161. doi:10.1046/j.0962-1083.2001.01363.x. PMID 11555258. http://www.life.illinois.edu/suarez/publications/Tsutsui_etal2001MolEcol.pdf. Retrieved 8 July 2017. https://www.life.illinois.edu/suarez/Publications/Tsutsui_etal2001MolEcol.pdf (dead)
- ↑ Roura-Pascual, Núria; Hui, Cang; Ikeda, Takayoshi; Leday, Gwénaël; Richardson, David M.; Carpintero, Soledad; Espadaler, Xavier; Gómez, Crisanto et al. (2011-01-04). "Relative roles of climatic suitability and anthropogenic influence in determining the pattern of spread in a global invader" (in en). Proceedings of the National Academy of Sciences 108 (1): 220–225. doi:10.1073/pnas.1011723108. ISSN 0027-8424. PMID 21173219. Bibcode: 2011PNAS..108..220R.
- ↑ "Linepithema humile" (in en). https://www.antweb.org/description.do?genus=linepithema&species=humile&rank=species.
- ↑ 8.0 8.1 Hartley, Stephen; Harris, Richard; Lester, Philip J. (2006-09-01). "Quantifying uncertainty in the potential distribution of an invasive species: climate and the Argentine ant" (in en). Ecology Letters 9 (9): 1068–1079. doi:10.1111/j.1461-0248.2006.00954.x. ISSN 1461-0248. PMID 16925656.
- ↑ Mothapo, Natasha P; Wossler, Theresa C (2011). "Behavioural and chemical evidence for multiple colonisation of the Argentine ant, Linepithema humile, in the Western Cape, South Africa". BMC Ecology 11 (1): 6. doi:10.1186/1472-6785-11-6. ISSN 1472-6785. PMID 21288369.
- ↑ Ward, D. F.; Green, C.; Harris, R. J.; Hartley, S.; Lester, P. J.; Stanley, M. C.; Suckling, D. M.; Toft, R. J. (2010). "Twenty years of Argentine ants in New Zealand: past research and future priorities for applied management". New Zealand Entomologist 33 (1): 68–78. doi:10.1080/00779962.2010.9722193. ISSN 0077-9962. http://dx.doi.org/10.1080/00779962.2010.9722193.
- ↑ Park, Sang-Hyun; Hosoishi, Shingo; Ogata, Kazuo (2014-08-28). "Long-term impacts of Argentine ant invasion of urban parks in Hiroshima, Japan". Journal of Ecology and Environment 37 (3): 123–129. doi:10.5141/ecoenv.2014.015. ISSN 2287-8327.
- ↑ Varela, Andrea Isabel; Luna, Nicolas; Luna-Jorquera, Guillermo (2018-05-02). "Assessing potential Argentine Ant recruitment to pipping eggs in the Red-tailed Tropicbird on Rapa Nui (Easter Island)". Emu - Austral Ornithology 118 (4): 381–385. doi:10.1080/01584197.2018.1464372. ISSN 0158-4197. http://dx.doi.org/10.1080/01584197.2018.1464372.
- ↑ Suhr, Elissa L.; O’Dowd, Dennis J.; McKechnie, Stephen W.; Mackay, Duncan A. (2010-12-06). "Genetic structure, behaviour and invasion history of the Argentine ant supercolony in Australia". Evolutionary Applications 4 (3): 471–484. doi:10.1111/j.1752-4571.2010.00161.x. ISSN 1752-4571. PMID 25567996. PMC 3352524. http://dx.doi.org/10.1111/j.1752-4571.2010.00161.x.
- ↑ Carpintero, S.; Retana, J.; Cerda, X.; Reyes-Lopez, J.; Arias de Reyna, L. (2007-10-01). "Exploitative Strategies of the Invasive Argentine Ant (Linepithema humile) and Native Ant Species in a Southern Spanish Pine Forest". Environmental Entomology 36 (5): 1100–1111. doi:10.1093/ee/36.5.1100. ISSN 0046-225X. PMID 18284734.
- ↑ Reimer, N.; Beardsley, J. W.; John, G. (2019). "Pest ants in the Hawaiian Islands". Applied Myrmecology. CRC Press. pp. 40–50.
- ↑ Holway, David A. (1995). "Distribution of the Argentine ant (Linepithema humile) in northern California". Conservation Biology 9 (6): 1634–1637. doi:10.1046/j.1523-1739.1995.09061634.x.
- ↑ Silverman, Jules; Brightwell, Robert John (2008-01-01). "The Argentine Ant: Challenges in Managing an Invasive Unicolonial Pest". Annual Review of Entomology 53 (1): 231–252. doi:10.1146/annurev.ento.53.103106.093450. ISSN 0066-4170. PMID 17877449. http://dx.doi.org/10.1146/annurev.ento.53.103106.093450.
- ↑ Rust, M. K.; Knight, R. L. (2019). "Controlling Argentine ants in urban situations". Applied Myrmecology. CRC Press. pp. 663–670.
- ↑ Moreno, D. S.; Haney, P. B.; Luck, R. F. (1987-02-01). "Chlorpyrifos and Diazinon as Barriers to Argentine Ant (Hymenoptera: Formicidae) Foraging on Citrus Trees". Journal of Economic Entomology 80 (1): 208–214. doi:10.1093/jee/80.1.208. ISSN 1938-291X. http://dx.doi.org/10.1093/jee/80.1.208.
- ↑ Cole, F. Russell; Medeiros, Arthur C.; Loope, Lloyd L.; Zuehlke, William W. (1992). "Effects of the Argentine Ant on Arthropod Fauna of Hawaiian High-Elevation Shrubland". Ecology 73 (4): 1313–1322. doi:10.2307/1940678. ISSN 0012-9658. http://dx.doi.org/10.2307/1940678.
- ↑ Holway, David A. (1998-08-10). "Effect of Argentine ant invasions on ground-dwelling arthropods in northern California riparian woodlands". Oecologia 116 (1–2): 252–258. doi:10.1007/s004420050586. ISSN 0029-8549. PMID 28308533. Bibcode: 1998Oecol.116..252H. http://dx.doi.org/10.1007/s004420050586.
- ↑ Lach, Lori (2007-08-20). "Argentine ants displace floral arthropods in a biodiversity hotspot". Diversity and Distributions 14 (2): 281–290. doi:10.1111/j.1472-4642.2007.00410.x. ISSN 1366-9516. http://dx.doi.org/10.1111/j.1472-4642.2007.00410.x.
- ↑ Suarez, A. V.; Yeh, P.; Case, T. J. (2005). "Impacts of Argentine ants on avian nesting success". Insectes Sociaux 52 (4): 378–382. doi:10.1007/s00040-005-0824-y. ISSN 0020-1812. http://dx.doi.org/10.1007/s00040-005-0824-y.
- ↑ Suarez, Andrew V.; Richmond, Jon Q.; Case, Ted J. (2000). [0711:psihlf2.0.co;2 "Prey Selection in Horned Lizards Following the Invasion of Argentine Ants in Southern California"]. Ecological Applications 10 (3): 711–725. doi:10.1890/1051-0761(2000)010[0711:psihlf2.0.co;2]. ISSN 1051-0761. http://dx.doi.org/10.1890/1051-0761(2000)010[0711:psihlf]2.0.co;2.
- ↑ 25.0 25.1 "Proliferation of Argentine ants in California linked to decline in coastal horned lizards". Science Daily (Press release). March 5, 2002.
- ↑ Bond, W.; Slingsby, P. (1984). "Collapse of an Ant-Plant Mutalism: The Argentine Ant (Iridomyrmex Humilis) and Myrmecochorous Proteaceae". Ecology 65 (4): 1031–1037. doi:10.2307/1938311. ISSN 0012-9658. http://dx.doi.org/10.2307/1938311.
- ↑ LeVan, Katherine E.; Hung, Keng-Lou James; McCann, Kyle R.; Ludka, John T.; Holway, David A. (2014). "Floral visitation by the Argentine ant reduces pollinator visitation and seed set in the coast barrel cactus, Ferocactus viridescens" (in en). Oecologia 174 (1): 163–171. doi:10.1007/s00442-013-2739-z. ISSN 0029-8549. PMID 23892582. Bibcode: 2014Oecol.174..163L. http://link.springer.com/10.1007/s00442-013-2739-z.
- ↑ LeVan, Katherine E.; Holway, David A. (2015). "Ant–aphid interactions increase ant floral visitation and reduce plant reproduction via decreased pollinator visitation" (in en). Ecology 96 (6): 1620–1630. doi:10.1890/14-0058.1. ISSN 0012-9658. http://doi.wiley.com/10.1890/14-0058.1.
- ↑ "Polygyny". https://www.antwiki.org/wiki/Polygyny.
- ↑ 30.0 30.1 Markin, George P. (1969). "The seasonal life cycle of the Argentine ant in southern California". Annals of the Entomological Society of America 63 (5): 1238. doi:10.1093/aesa/63.5.1238.
- ↑ Scholes, D. R.; Suarez, A. V. (2009-08-30). "Speed-versus-accuracy trade-offs during nest relocation in Argentine ants (Linepithema humile) and odorous house ants (Tapinoma sessile)". Insectes Sociaux 56 (4): 413–418. doi:10.1007/s00040-009-0039-8. ISSN 0020-1812. http://dx.doi.org/10.1007/s00040-009-0039-8.
- ↑ Markin, George P. (1970-09-15). "The Seasonal Life Cycle of the Argentine Ant, Iridomyrmex humilis (Hymenoptera: Formicidae), in Southern California". Annals of the Entomological Society of America 63 (5): 1238–1242. doi:10.1093/aesa/63.5.1238. ISSN 1938-2901. http://dx.doi.org/10.1093/aesa/63.5.1238.
- ↑ Alexander L. Wild (2004). "Taxonomy and distribution of the Argentine ant, Linepithema humile (Hymenoptera: Formicidae)" (PDF). Annals of the Entomological Society of America 97 (6): 1204–1215. doi:10.1603/0013-8746(2004)097[1204:TADOTA2.0.CO;2]. https://archive.org/details/ants_20351.
- ↑ 34.0 34.1 Tsutsui, Neil D.; Suarez, Andrew V.; Holway, David A.; Case, Ted J. (2000). "Reduced genetic variation and the success of an invasive species". Proceedings of the National Academy of Sciences 97 (11): 5948–5953. doi:10.1073/pnas.100110397. PMID 10811892. Bibcode: 2000PNAS...97.5948T.
- ↑ Andrew V. Suarez; David A. Holway; Ted J. Case (2001). "Patterns of spread in biological invasions dominated by long-distance jump dispersal: Insights from Argentine ants". Proceedings of the National Academy of Sciences 98 (3): 1095–1100. doi:10.1073/pnas.98.3.1095. PMID 11158600. Bibcode: 2001PNAS...98.1095S.
- ↑ Newell, Wilmon; Barber, T.C. (1913). "The Argentine ant". USDA Bureau of Entomology Bulletin 122: 1–98.
- ↑ Giraud, Tatiana; Pedersen, Jes S.; Keller, Laurent (2002). "Evolution of supercolonies: The Argentine ants of southern Europe". Proceedings of the National Academy of Sciences 99 (9): 6075–6079. doi:10.1073/pnas.092694199. PMID 11959924. Bibcode: 2002PNAS...99.6075G.
- ↑ 38.0 38.1 38.2 Sunamura, E.; Espadaler, X.; Sakamoto, H.; Suzuki, S.; Terayama, M.; Tatsuki, S. (2009-03-04). "Intercontinental union of Argentine ants: Behavioral relationships among introduced populations in Europe, North America, and Asia". Insectes Sociaux 56 (2): 143–147. doi:10.1007/s00040-009-0001-9. ISSN 0020-1812.
- ↑ Suhr, Elissa; O’Dowd, Dennis J.; McKechnie, Stephen W.; Mackay, Duncan A. (2010). "Genetic structure, behaviour and invasion history of the Argentine ant supercolony in Australia". Evolutionary Applications 4 (3): 471–484. doi:10.1111/j.1752-4571.2010.00161.x. PMID 25567996.
- ↑ van Wilgenburg, Ellen; Torres, Candice W.; Tsutsui, Neil D. (2010). "The global expansion of a single ant supercolony". Evolutionary Applications 3 (2): 136–143. doi:10.1111/j.1752-4571.2009.00114.x. PMID 25567914.
- ↑ Giraud, Tatiana; Pedersen, Jes S.; Keller, Laurent (2002). "Evolution of supercolonies: the Argentine ants of southern Europe". Proceedings of the National Academy of Sciences 99 (9): 6075–6079. doi:10.1073/pnas.092694199. PMID 11959924. Bibcode: 2002PNAS...99.6075G.
- ↑ 42.0 42.1 Moffett, Mark (2010). Adventures Among Ants: A global safari with a cast of trillions. Berkeley and Los Angeles, CA: University of California Press. pp. 203–205. ISBN 978-0-520-26199-0. https://archive.org/details/adventuresamonga00moff/page/203.
- ↑ Success of Introduced Argentine Ants Tied to Reduced Genetic Variation. Kim McDonald, University of California, May 2000
- ↑ Buczkowski, Grzegorz; Kumar, Ranjit; Suib, Steven L.; Silverman, Jules (2005-05-25). "Diet-related modification of cuticular hydrocarbon profiles of the Argentine ant, Linepithema humile, diminishes intercolony aggression". Journal of Chemical Ecology 31 (4): 829–843. doi:10.1007/s10886-005-3547-7. PMID 16124254.
- ↑ Corin, S.E.; Abbott, K.L.; Ritchie, P.A.; Lester, P.J. (2007-06-12). "Large scale unicoloniality: The population and colony structure of the invasive Argentine ant (Linepithema humile) in New Zealand". Insectes Sociaux 54 (3): 275–282. doi:10.1007/s00040-007-0942-9.
- ↑ Passera, L.; Keller, L.; Suzzoni, J.P. (March 1988). "Control of brood male production in the Argentine ant Iridomyrmex humilis (Mayr)". Insectes Sociaux 35 (1): 19–33. doi:10.1007/BF02224135.
- ↑ 47.0 47.1 Heller, Nicole E.; Sanders, Nathan J.; Gordon, Deborah M. (2006). "Linking temporal and spatial scales in the study of an Argentine ant invasion". Biological Invasions 8 (3): 501–507. doi:10.1007/s10530-005-6411-3.
- ↑ Boudjelas, Souyad (2000). 100 of the world's worst invasive alien species (Report). International Union for Conservation of Nature. https://portals.iucn.org/library/sites/library/files/documents/2000-126.pdf. Retrieved 20 July 2018.
- ↑ Bristow, C.M. (1991). "Are ant–aphid associations a tritrophic interaction? Oleander aphids and Argentine ants". Oecologia 87 (4): 514–521. doi:10.1007/BF00320414. PMID 28313693. Bibcode: 1991Oecol..87..514B.
- ↑ Grover, Crystal D.; Dayton, Kathleen C.; Menke, Sean B.; Holway, David A. (2008). "Effects of aphids on foliar foraging by Argentine ants and the resulting effects on other arthropods". Ecological Entomology 33 (1): 101–106. doi:10.1111/j.1365-2311.2007.00942.x. http://www4.ncsu.edu/~sbmenke/Publications/Grover_et_al%20Ecol%20Ent%202008.pdf.
- ↑ Sahli, Heather F.; Krushelnycky, Paul D.; Drake, Donald R.; Taylor, Andrew D. (Jul 2016). "Patterns of floral visitation to native Hawaiian plants in presence and absence of invasive Argentine ants". Pacific Science 70 (3): 309–322. doi:10.2984/70.3.3. ISSN 0030-8870.
- ↑ Klotz, John H.; Greenberg, Les; Amrhein, Christopher; Rust, Michael K. (August 2000). "Toxicity and Repellency of Borate-Sucrose Water Baits to Argentine Ants (Hymenoptera: Formicidae)". Journal of Economic Entomology 93 (4): 1256–8. doi:10.1603/0022-0493-93.4.1256. PMID 10985039. http://www.urban.ucr.edu/docs/Argentine%20Ant/2000%20Klotz%20et%20al.%20Toxicity%20and%20Repellency%20of%20Borate-Sucrose%20.pdf. Retrieved 2017-01-15.
- ↑ Klotz, John H.; Rust, Michael K.; Amrhein, Christopher; Krieger, Robert (September 2004). "In search of the 'sweet spot'". Pest Control. http://urban.ucr.edu/docs/Argentine%20Ant/2004%20Klotz%20et%20al.%20In%20Search%20of%20the%20SweetSpot.pdf. Retrieved 2017-01-15.
- ↑ Abril, S.; Oliveras, J.; Gómez, C. (Oct 2007). "Foraging activity and dietary spectrum of the Argentine ant (Hymenoptera: Formicidae) in invaded natural areas of the northeast Iberian Peninsula". Environmental Entomology 36 (5): 1166–1173. doi:10.1603/0046-225X(2007)36[1166:FAADSO2.0.CO;2]. ISSN 0046-225X. PMID 18284742.
- ↑ Perlman, David (2006-09-15). "Ants' own chemical may control them". SFGate. http://sfgate.com/cgi-bin/article.cgi?f=/c/a/2006/09/15/BAG75L6BJK1.DTL.
- ↑ Rivenburg, Roy (September 15, 2006). "UCI makes ants go ape by giving them B.O.". Los Angeles Times. http://articles.latimes.com/2006/sep/15/local/me-ants15.
- ↑ ; Mamoru Terayama & Yasutoshi Tanaka et al."Behavior-disrupting agent and behavior-disrupting method of Argentine ant" US patent 8278360, published 2012-10-02, assigned to Shin-Etsu Chemical Co Ltd
- ↑ David M Suckling; R. W. Peck; L. M. Manning; L. D. Stringer; J. Cappadonna; A. M. El-Sayed (2008). "Disruption of Foraging by a Dominant Invasive Species to Decrease Its Competitive Ability". Journal of Chemical Ecology 34 (12): 1602–9. doi:10.1007/s10886-008-9566-4. PMID 19034574.
- ↑ Fabian L. Westermann; David M. Suckling; Philip J. Lester (2014). "Disruption of Foraging by a Dominant Invasive Species to Decrease Its Competitive Ability". PLOS One 9 (3): e90173. doi:10.1371/journal.pone.0090173. PMID 24594633. Bibcode: 2014PLoSO...990173W.
External links
- National Pest Management Association. "Argentine Ant Fact Sheet". http://www.pestworld.org/pest-guide/ants/argentine-ants/. with information on habits, habitat and prevention
- Matt Daugherty. "Argentine ant (Linepithema humile)". Center for Invasive Species Research. University of California, Riverside. http://www.cisr.ucr.edu/argentine_ant.html.
- Alex Wild (2008-04-13). "How to identify the Argentine ant, Linepithema humile". Myrmecos. http://myrmecos.net/2008/04/13/how-to-identify-the-argentine-ant-linepithema-humile/.
Wikidata ☰ Q645538 entry
 |