Chemistry:Hydramethylnon
| |
| Names | |
|---|---|
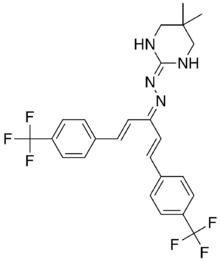
| IUPAC name
2(1H-4,4-dimethyl tetrahydro pyrimidinylidene )
(3-(4-(trifluoromethyl)phenyl) -1-(2-(4-(trifluoromethyl)phenyl)ethenyl) -2-propenylidene)hydrazone | |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| KEGG | |
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| C25H24F6N4 | |
| Molar mass | 494.485 g·mol−1 |
| Appearance | Yellow to orange crystalline solid |
| Melting point | 185 to 190 °C (365 to 374 °F; 458 to 463 K) |
| Hazards | |
| NFPA 704 (fire diamond) | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Hydramethylnon is an organofluorine compound. It is also known as AC 217,300. It is in a chemical class called trifluoromethyl aminohydrazone, which is a metabolic inhibitor. It is classified as a pesticide designed to control insects that are harmful to humans.[1] It works by inhibiting complex III in the mitochondrial inner membrane and leads to a halting of oxidative phosphorylation. It is used primarily as an insecticide in the form of baits for cockroaches and ants.[2] Some brands of insecticides that include hydramethylnon are Amdro, Blatex, Combat, Cyaforce, Cyclon, Faslane, Grant's, Impact, Matox, Maxforce, Pyramdron, Siege, Scuttle and Wipeout. Hydramethylnon is a slow-acting poison with delayed toxicity that needs to be eaten to be effective.[3]
Toxicology
Hydramethylnon has low toxicity in mammals.[2][3] The oral -1">50 is 1100–1300 mg/kg in rats and above 28,000 mg/kg in dogs.[3] Hydramethylnon is toxic to fish; the 96-hour LC50 in rainbow trout is 0.16 mg/L, 0.10 mg/L in channel catfish, and 1.70 mg/L in bluegill sunfish.
Hydramethylnon, when fed to rats for two years, led to an increase in uterine and adrenal tumors at the highest dose; therefore, the Environmental Protection Agency classifies hydramethylnon as a possible human carcinogen.[3]
See also
- Fipronil, another insecticide used for similar purposes
References
- ↑ "Hydramethylnon". PubChem. https://pubchem.ncbi.nlm.nih.gov/compound/hydramethylnon#section=Pharmacology-and-Biochemistry.
- ↑ 2.0 2.1 Veterinary Support Personnel Network
- ↑ 3.0 3.1 3.2 3.3 "Hydramethylnon". National Pesticide Information Center. http://npic.orst.edu/factsheets/hydragen.pdf.
External links
- Hydramethylnon in the Pesticide Properties DataBase (PPDB)
- Hydramethylnon Technical Fact Sheet - National Pesticide Information Center
- Hydramethylnon General Fact Sheet - National Pesticide Information Center
- Hydramethylnon Pesticide Information Profile - Extension Toxicology Network
- Maxforce MSDS.
- Amdro MSDS
 |

