Biology:MiR-324-5p
miR-324-5p is a microRNA that functions in cell growth, apoptosis, cancer,[1] epilepsy,[2][3] neuronal differentiation,[4] psychiatric conditions,[5] cardiac disease pathology,[6][1] and more.[7] As a microRNA, it regulates gene expression through targeting mRNAs. Additionally, miR-324-5p is both an intracellular miRNA, meaning it is commonly found within the microenvironment of the cell, and one of several circulating miRNAs found throughout the body.[8] Its presence throughout the body both within and external to cells may contribute to miR-324-5p's wide array of functions and role in numerous disease pathologies – especially cancer – in various organ systems.
History
miR-324-5p first appeared in literature in a paper published by John Kim et al. in early 2004 that identified 32 entirely new miRNAs from cultured rat cortical neurons using miRNA cloning and RNA analysis.[9] The miRNA quickly gained traction in scientific literature, appearing in articles about the evolutionary conservation of microRNAs,[10] HIV,[11] cancer,[12] and other topics within a few years. Today, the functions and roles of miR-324-5p are still not yet fully characterized.[13]
Structure and targets
miR-324-5p is a reverse strand miRNA, meaning it is produced from the 5' end of the associated RNA, and spans from position 7,223,342 to 7,223,364 on chromosome 17.[14] Its sequence is CGCAUCCCCUAGGGCAUUGGUG.[15][16]
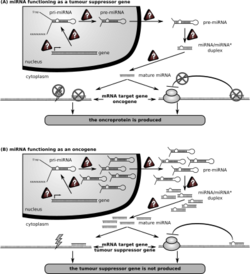
miRNA forms following cleavage of pre-miRNA at the hairpin loop by the enzyme dicer within the cytosol. Interestingly, both strands of miR-324's pre-miRNA hairpin loop structure, miR-324-5p and miR-324-3p, become active miRNAs with distinct targets and functions.[17] miR-324-5p has between 166 and 469 predicted targets,[18][19][14] including regulators of cell growth, proliferation, survival, cytoskeletal structure, ATP transport, and ion channels.[18] Though miR-324-5p is found on chromosome 17, its targets span across all chromosomes.[16]
Functions
Cell growth and survival
miR-324-5p likely regulates cell growth and survival through interaction with multiple pathways. Published research demonstrates that this miRNA interacts with the Hedgehog (HH) signaling pathway via interactions with HH transcription factor Gli1[20] and HH protein receptor Smo,[21] often contributing to tumorigenesis. miR-324-5p's activating interaction with the protein NfkB also regulates numerous components of cell survival, including cell cycle control, enzyme synthesis, and cell adhesion.[22] In addition, miR-324-5p regulates components of the MAPK pathway, influencing cell growth, proliferation, and survival. Specifically, miR-324-5p downregulates RAF and ERK and is necessary for normal levels of cell growth. Reduced expression leads to increased cell growth and proliferation, and overexpression limits growth, leading to its role in oncogenesis.[23]
Cancer
Both up and downregulation of miR-324-5p is shown to contribute to various types of cancer.[17]
miR-324-5p plays a role in inflammation and tumorigenesis in colorectal cancer through regulation of CUEDC2, which regulates inflammation via interaction with NF-kB signaling.[12] miR-324-5p can inhibit glioma proliferation,[20] suppress hepatocellular carcinoma and nasopharyngeal carcinoma cell invasion,[24][25] and regulate growth and pathology in multiple myeloma.[26] Additionally, chromosome 17 deletions, which include deletion of miR-324-5p, are present in 10% of multiple myeloma patients and are associated with poorer prognosis.[26]
In contrast, overexpression of miR-324-5p in gastric cancer cells reduces cell death and promotes growth and proliferation.[27] miR-324-5p has also been shown to reduce the viability of gastric cancer cells via downregulation of TSPAN8, and miR-324-5p expression increased apoptosis in these same gastric cancer cells.[28]
Epilepsy
Seizures are characterized by high levels of synchronized neuronal activity. One important regulator of neuronal activity is the hyperpolarizing A-type current mediated by potassium channel KV4.2.[29] miR-324-5p downregulates KV4.2, exacerbating conditions that lead to seizure onset, and downregulation of miR-324-5p in mouse models of epilepsy is seizure-suppressive.[3]
Changes in miRNA expression are seen in epileptogenesis and in other disease pathologies.[30][31] In epilepsy, miR-324-5p expression has been shown to increase[32] and decrease[33] at different timepoints and loci.
Importantly, miR-324-5p has increased association with the RISC complex following seizure in mice, indicating more suppressive activity.[3][34]
Overall, this suggests that miR-324-5p plays a role in epileptogenesis via targeting of potassium channel KV4.2.
Cardiac disease
miR-324-5p contributes to cardiac disease pathophysiology and cardiomyocite death through translational inhibition of Mtfr1, leading to reduced mitochondrial fission, apoptosis, and myocardial infarction.[6]
Psychiatric conditions
MiRNA expression profiles are altered in psychiatric conditions, including depression,[5] anxiety,[35] and PTSD.[36] It has been demonstrated that miR-324-5p expression is altered in the brains of suicide victims with depression[5] and in the amygdala, the fear center of the brain, in PTSD.[36] MiRNAs are an underexplored potential biomarker and target for treatment for psychiatric disease.[37]
Future research and potential in medicine
miRNA-324-5p is a relatively new and understudied microRNA. It is an important regulator in several diseases, and its effects span across the body from neuronal dysregulation in seizure to hepatocellular carcinoma and cardiac disease. Because microRNAs have numerous targets, they are capable of regulating multiple pathways and circuits, an ability that may be useful in the treatment of complex disorders like epilepsy in which many subsystems are dysregulated. However, the wide-ranging functions of miRNAs may be limiting as well. microRNA expression modulation could lead to unanticipated physiological effects and not provide adequate specificity.[38]
References
- ↑ 1.0 1.1 "Cardio-miRNAs and onco-miRNAs: circulating miRNA-based diagnostics for non-cancerous and cancerous diseases". Frontiers in Cell and Developmental Biology 2: 61. 2014. doi:10.3389/fcell.2014.00061. PMID 25364765.
- ↑ Yao X (2012). Regulation of A-type potassium channel Kv4.2 expression by FMRP and miR-324-5p (Ph.D. thesis). Emory University.
- ↑ 3.0 3.1 3.2 "MicroRNA-Mediated Downregulation of the Potassium Channel Kv4.2 Contributes to Seizure Onset". Cell Reports 17 (1): 37–45. September 2016. doi:10.1016/j.celrep.2016.08.074. PMID 27681419.
- ↑ "MicroRNA-based promotion of human neuronal differentiation and subtype specification". PLOS ONE 8 (3): e59011. 2013. doi:10.1371/journal.pone.0059011. PMID 23527072. Bibcode: 2013PLoSO...859011S.
- ↑ 5.0 5.1 5.2 "MicroRNA expression is down-regulated and reorganized in prefrontal cortex of depressed suicide subjects". PLOS ONE 7 (3): e33201. 2012. doi:10.1371/journal.pone.0033201. PMID 22427989. Bibcode: 2012PLoSO...733201S.
- ↑ 6.0 6.1 "NFAT4-dependent miR-324-5p regulates mitochondrial morphology and cardiomyocyte cell death by targeting Mtfr1". Cell Death & Disease 6 (12): e2007. December 2015. doi:10.1038/cddis.2015.348. PMID 26633713.
- ↑ "MicroRNA screening identifies a link between NOVA1 expression and a low level of IKAP in familial dysautonomia". Disease Models & Mechanisms 9 (8): 899–909. August 2016. doi:10.1242/dmm.025841. PMID 27483351.
- ↑ "Circulating microRNAs as candidate markers to distinguish heart failure in breathless patients". European Journal of Heart Failure 15 (10): 1138–47. October 2013. doi:10.1093/eurjhf/hft078. PMID 23696613.
- ↑ "Identification of many microRNAs that copurify with polyribosomes in mammalian neurons". Proceedings of the National Academy of Sciences of the United States of America 101 (1): 360–5. January 2004. doi:10.1073/pnas.2333854100. PMID 14691248. Bibcode: 2004PNAS..101..360K.
- ↑ "Evolutionary patterns of non-coding RNAs". Theory in Biosciences = Theorie in den Biowissenschaften 123 (4): 301–69. April 2005. doi:10.1016/j.thbio.2005.01.002. PMID 18202870.
- ↑ "Targets for human encoded microRNAs in HIV genes". Biochemical and Biophysical Research Communications 337 (4): 1214–8. December 2005. doi:10.1016/j.bbrc.2005.09.183. PMID 16236258.
- ↑ 12.0 12.1 "Dysregulation of the miR-324-5p-CUEDC2 axis leads to macrophage dysfunction and is associated with colon cancer". Cell Reports 7 (6): 1982–93. June 2014. doi:10.1016/j.celrep.2014.05.007. PMID 24882011.
- ↑ "MIR324 microRNA 324 [Homo sapiens (human) - Gene - NCBI"] (in en). https://www.ncbi.nlm.nih.gov/gene/442898.
- ↑ 14.0 14.1 "Homo sapiens (human) hsa-miR-324-5p | URS000005481D". RNAcentral. European Molecular Biology Laboratory. https://rnacentral.org/rna/URS000005481D/9606.
- ↑ "MiRNA Entry for MI0000813". miRBase: the microRNA database. The University of Manchester. http://mirbase.org/cgi-bin/mirna_entry.pl?acc=MI0000813.
- ↑ 16.0 16.1 "miRNA ID: hsa-miR-324-5p". TargetMiner: Prediction of miRNA Targets. Indian Statistical Institute. https://www.isical.ac.in/~bioinfo_miu/final_html_targetminer/hsa-miR-324-5p.html.
- ↑ 17.0 17.1 "MicroRNA-324 in Human Cancer: miR-324-5p and miR-324-3p Have Distinct Biological Functions in Human Cancer". Anticancer Research 36 (10): 5189–5196. October 2016. doi:10.21873/anticanres.11089. PMID 27798879.
- ↑ 18.0 18.1 Wang, Xiaowei. "predicted targets for hsa-miR-324-5p in miRDB". miRDB: predicted microRNA targets in animals. St. Louis: Department of Radiation Oncology, Washington University School of Medicine. http://mirdb.org/cgi-bin/search.cgi?searchType=miRNA&full=mirbase&searchBox=MIMAT0000761.
- ↑ "Predicted miRNA targets of miR-324-5p". TargetscanHuman 7.1. Whitehead Institute for Biomedical Research. http://www.targetscan.org/cgi-bin/targetscan/vert_71/targetscan.cgi?mirg=hsa-miR-324-5p.
- ↑ 20.0 20.1 "MiR-324-5p inhibits proliferation of glioma by target regulation of GLI1". European Review for Medical and Pharmacological Sciences 18 (6): 828–32. 2014-03-30. PMID 24706306. http://www.europeanreview.org/article/7143.
- ↑ "Concerted microRNA control of Hedgehog signalling in cerebellar neuronal progenitor and tumour cells". The EMBO Journal 27 (19): 2616–27. October 2008. doi:10.1038/emboj.2008.172. PMID 18756266.
- ↑ "Sinomenine inhibits breast cancer cell invasion and migration by suppressing NF-κB activation mediated by IL-4/miR-324-5p/CUEDC2 axis". Biochemical and Biophysical Research Communications 464 (3): 705–10. August 2015. doi:10.1016/j.bbrc.2015.07.004. PMID 26166821.
- ↑ "miR-524-5p suppresses the growth of oncogenic BRAF melanoma by targeting BRAF and ERK2". Oncotarget 5 (19): 9444–59. October 2014. doi:10.18632/oncotarget.2452. PMID 25275294.
- ↑ "MiR-324-5p Suppresses Hepatocellular Carcinoma Cell Invasion by Counteracting ECM Degradation through Post-Transcriptionally Downregulating ETS1 and SP1". PLOS ONE 10 (7): e0133074. 2015. doi:10.1371/journal.pone.0133074. PMID 26177288. Bibcode: 2015PLoSO..1033074C.
- ↑ "miR-324-3p suppresses migration and invasion by targeting WNT2B in nasopharyngeal carcinoma". Cancer Cell International 17: 2. 2017. doi:10.1186/s12935-016-0372-8. PMID 28053597.
- ↑ 26.0 26.1 "MicroRNA-324-5p regulates stemness, pathogenesis and sensitivity to bortezomib in multiple myeloma cells by targeting hedgehog signaling". International Journal of Cancer 142 (1): 109–120. January 2018. doi:10.1002/ijc.31041. PMID 28905994.
- ↑ "miR-324-3p promotes gastric cancer development by activating Smad4-mediated Wnt/beta-catenin signaling pathway". Journal of Gastroenterology 53 (6): 725–739. June 2018. doi:10.1007/s00535-017-1408-0. PMID 29103082.
- ↑ "MiR-324-5p reduces viability and induces apoptosis in gastric cancer cells through modulating TSPAN8". The Journal of Pharmacy and Pharmacology 70 (11): 1513–1520. November 2018. doi:10.1111/jphp.12995. PMID 30159900.
- ↑ "Role of A-type potassium currents in excitability, network synchronicity, and epilepsy". Hippocampus 20 (7): 877–87. July 2010. doi:10.1002/hipo.20694. PMID 19777555.
- ↑ "microRNAs in the pathophysiology of epilepsy". Neuroscience Letters 667: 47–52. February 2018. doi:10.1016/j.neulet.2017.01.017. PMID 28104433.
- ↑ "MicroRNAs in common human diseases". Genomics, Proteomics & Bioinformatics 10 (5): 246–53. October 2012. doi:10.1016/j.gpb.2012.07.005. PMID 23200134.
- ↑ "MicroRNA-124 and -137 cooperativity controls caspase-3 activity through BCL2L13 in hippocampal neural stem cells". Scientific Reports 5: 12448. July 2015. doi:10.1038/srep12448. PMID 26207921. Bibcode: 2015NatSR...512448S.
- ↑ "Alterations in miRNA levels in the dentate gyrus in epileptic rats". PLOS ONE 8 (10): e76051. 2013. doi:10.1371/journal.pone.0076051. PMID 24146813. Bibcode: 2013PLoSO...876051B.
- ↑ "MicroRNA-induced silencing in epilepsy: Opportunities and challenges for clinical application". Developmental Dynamics 247 (1): 94–110. January 2018. doi:10.1002/dvdy.24582. PMID 28850760.
- ↑ "MicroRNA Regulators of Anxiety and Metabolic Disorders". Trends in Molecular Medicine 22 (9): 798–812. September 2016. doi:10.1016/j.molmed.2016.07.001. PMID 27496210.
- ↑ 36.0 36.1 "Serum and amygdala microRNA signatures of posttraumatic stress: fear correlation and biomarker potential". Journal of Psychiatric Research 57: 65–73. October 2014. doi:10.1016/j.jpsychires.2014.05.020. PMID 24998397.
- ↑ "MicroRNAs as Biomarkers for Psychiatric Conditions: A Review of Current Research". Innovations in Clinical Neuroscience 14 (1–2): 53–55. 2017. PMID 28386521.
- ↑ "Multiple-to-multiple relationships between microRNAs and target genes in gastric cancer". PLOS ONE 8 (5): e62589. 2013-05-08. doi:10.1371/journal.pone.0062589. PMID 23667495. Bibcode: 2013PLoSO...862589H.
 |