Medicine:Hepatocellular carcinoma
| Hepatocellular carcinoma | |
|---|---|
| Other names | Hepatoma |
 | |
| Hepatocellular carcinoma in an individual who was hepatitis C positive. Autopsy specimen. | |
| Specialty | Oncology |
Hepatocellular carcinoma (HCC[1]) is the most common type of primary liver cancer in adults and is currently the most common cause of death in people with cirrhosis.[2] HCC is the third leading cause of cancer-related deaths worldwide.[3]
The development of HCC is attributed to fibrosis and cirrhosis, which occur in the setting of chronic liver injury and inflammation. The latter being closely linked to chronic viral hepatitis infection (hepatitis B or C) or exposure to toxins such as alcohol, aflatoxin, or pyrrolizidine alkaloids.[4] Certain diseases, such as hemochromatosis and alpha 1-antitrypsin deficiency, markedly increase the risk of developing HCC. Metabolic syndrome and NASH are also increasingly recognized as risk factors for HCC.[5]:870–873
As with any cancer, the treatment and prognosis of HCC vary depending on the specifics of tumor histology, size, how far the cancer has spread, and overall health.
The vast majority of HCC cases and the lowest survival rates after treatment occur in Asia and sub-Saharan Africa, in countries where hepatitis B infection is endemic and many are infected from birth. The incidence of HCC in the United States and other developing countries is increasing due to an increase in hepatitis C virus infections. It is more than three times as common in males as in females, for unknown reasons.[5]:870–873
Signs and symptoms
Most cases of HCC occur in people who already have signs and symptoms of chronic liver disease. They may present with worsening symptoms or without symptoms at the time of cancer detection. HCC may present with non-specific symptoms such as abdominal pain, nausea, vomiting, or feeling tired.[6] Some symptoms that are more closely associated with liver disease include yellow skin (also called jaundice), abdominal swelling due to fluid in the abdominal cavity, easy bruising from blood clotting abnormalities, loss of appetite, unintentional weight loss, abdominal pain, nausea, vomiting, or feeling tired.[6]
Risk factors
Since HCC mostly occurs in people with cirrhosis of the liver, risk factors generally include factors which cause chronic liver disease that may lead to cirrhosis. Still, certain risk factors are more highly associated with HCC than others. For example, while heavy alcohol consumption is estimated to cause 60–70% of cirrhosis, the vast majority of HCC occurs in cirrhosis attributed to viral hepatitis (although there may be overlap).[7] Recognized risk factors include:
- Chronic viral hepatitis (estimated cause of 80% cases globally)
- Chronic hepatitis B (about 50% cases)
- Chronic hepatitis C (about 25% cases)[8]
- Toxins:
- Metabolic:
- Nonalcoholic steatohepatitis: up to 20% progress to cirrhosis [9]
- Nonalcoholic fatty liver disease[10]
- Type 2 diabetes (probably aided by obesity)[11]
- Congenital disorders:
- Alpha 1-antitrypsin deficiency
- Wilson's disease (controversial; while some theorise the risk increases,[12] case studies are rare[13] and suggest the opposite where Wilson's disease actually may confer protection[14])
- Hemophilia, although statistically associated with higher risk of HCC,[15] this is due to coincident chronic viral hepatitis infection related to repeated blood transfusions over lifetime.[1]
The significance of these risk factors varies globally. In regions where hepatitis B infection is endemic, such as southeast China, hepatitis B is the predominant cause.[16] In populations largely protected by hepatitis B vaccination, such as the United States, HCC is most often linked to causes of cirrhosis such as chronic hepatitis C, obesity, and excessive alcohol use.[17]
Certain benign liver tumors, such as hepatocellular adenoma, may sometimes be associated with coexisting malignant HCC. Evidence is limited for the true incidence of malignancy associated with benign adenomas; however, the size of hepatic adenoma is considered to correspond to risk of malignancy and so larger tumors may be surgically removed. Certain subtypes of adenoma, particularly those with β-catenin activation mutation, are particularly associated with increased risk of HCC.[17]
Chronic liver disease is rare in children and adolescents; however, congenital liver disorders are associated with an increased the chance of developing HCC.[18] Specifically, children with biliary atresia, infantile cholestasis, glycogen-storage diseases, and other cirrhotic diseases of the liver are predisposed to developing HCC in childhood.[citation needed]
Young adults afflicted by the rare fibrolamellar variant of hepatocellular carcinoma may have none of the typical risk factors, such as cirrhosis and hepatitis.[17]
Diabetes mellitus
The risk of hepatocellular carcinoma in type 2 diabetics is greater (from 2.5[11] to 7.1[19] times the nondiabetic risk) depending on the duration of diabetes and treatment protocol.[20] A suspected contributor to this increased risk is circulating insulin concentration such that diabetics with poor insulin control or on treatments that elevate their insulin output (both states that contribute to a higher circulating insulin concentration) show far greater risk of hepatocellular carcinoma than diabetics on treatments that reduce circulating insulin concentration.[11][19][21][22] On this note, some diabetics who engage in tight insulin control (by keeping it from being elevated) show risk levels low enough to be indistinguishable from the general population.[19][21] This phenomenon is thus not isolated to diabetes mellitus type 2, since poor insulin regulation is also found in other conditions such as metabolic syndrome (specifically, when evidence of nonalcoholic fatty liver disease or NAFLD is present) and again evidence of greater risk exists here, too.[23][24] While there are claims that anabolic steroid abusers are at greater risk[25] (theorized to be due to insulin and IGF exacerbation[26][27]), the only evidence that has been confirmed is that anabolic steroid users are more likely to have the benign hepatocellular adenomas transform into the more dangerous hepatocellular carcinoma.[28][29]
Pathogenesis
Hepatocellular carcinoma, like any other cancer, develops when epigenetic alterations and mutations affecting the cellular machinery cause the cell to replicate at a higher rate and/or result in the cell avoiding apoptosis.[30]
In particular, chronic infections of hepatitis B and/or C can aid the development of hepatocellular carcinoma by repeatedly causing the body's own immune system to attack the liver cells, some of which are infected by the virus, others merely bystanders.[31] Activated immune-system inflammatory cells release free radicals, such as reactive oxygen species and nitric oxide reactive species, which in turn can cause DNA damage and lead to carcinogenic gene mutations.[32] Reactive oxygen species also cause epigenetic alterations at the sites of DNA repair.[33]
While this constant cycle of damage followed by repair can lead to mistakes during repair, which in turn lead to carcinogenesis, this hypothesis is more applicable, at present, to hepatitis C. Chronic hepatitis C causes HCC through the stage of cirrhosis. In chronic hepatitis B, however, the integration of the viral genome into infected cells can directly induce a noncirrhotic liver to develop HCC. Alternatively, repeated consumption of large amounts of ethanol can have a similar effect. The toxin aflatoxin from certain Aspergillus species of fungi is a carcinogen and aids carcinogenesis of hepatocellular cancer by building up in the liver. The combined high prevalence of rates of aflatoxin and hepatitis B in settings such as China and West Africa has led to relatively high rates of hepatocellular carcinoma in these regions. Other viral hepatitides such as hepatitis A have no potential to become a chronic infection, thus are not related to HCC.[17]
Diagnosis
Methods of diagnosis in HCC have evolved with the improvement in medical imaging. The evaluation of both asymptomatic patients and those with symptoms of liver disease involves blood testing and imaging evaluation. Historically, a biopsy of a tumor was required to prove an HCC diagnosis. However, imaging (especially MRI) findings may be conclusive enough without histopathologic confirmation.[17]
Screening
HCC remains associated with a high mortality rate, in part because initial diagnosis commonly occurs at an advanced stage of disease. As with other cancers, outcomes are significantly improved if treatment is initiated earlier in the disease process. Since the vast majority of HCC cases occur in people with certain chronic liver diseases, especially those with cirrhosis, liver screening is commonly advocated in this population. Specific screening guidelines continue to evolve over time as evidence of its clinical impact becomes available. In the United States, the most commonly observed guidelines are those published by the American Association for the Study of Liver Diseases(AASLD), which recommends ultrasound screenings every six months for people with cirrhosis, with or without measurement of blood levels of tumor marker alpha-fetoprotein (AFP).[34] Elevated levels of AFP are associated with active HCC disease, though their reliability can be inconsistent. At levels >20, sensitivity is 41–65% and specificity is 80–94%. However, at levels >200, sensitivity is 31 and specificity is 99%.[35]
On ultrasound, HCC often appears as a small hypoechoic lesion with poorly defined margins and coarse, irregular internal echoes. When the tumor grows, it can sometimes appear heterogeneous with fibrosis, fatty change, and calcifications. This heterogeneity can look similar to cirrhosis and the surrounding liver parenchyma. A systematic review found that the sensitivity was 60% (95% CI 44–76%) and specificity was 97% (95% CI 95–98%) compared with pathologic examination of an explanted or resected liver as the reference standard. The sensitivity increases to 79% with AFP correlation.[36]
Controversy remains as to the most effective screening protocols. For example, while some data support decreased mortality related to screening people with hepatitis B infection, the AASLD notes, “There are no randomized trials [for screening] in Western populations with cirrhosis secondary to chronic hepatitis C or fatty liver disease, and thus there is some controversy surrounding whether surveillance truly leads to a reduction in mortality in this population of patients with cirrhosis.”[34]
Higher risk people
In a person where a higher suspicion of HCC exists, such as a person with symptoms or abnormal blood tests (i.e. alpha-fetoprotein and des-gamma carboxyprothrombin levels),[37] evaluation requires imaging of the liver by CT or MRI scans. Optimally, these scans are performed with intravenous contrast in multiple phases of hepatic perfusion to improve detection and accurate classification of any liver lesions by the interpreting radiologist. Due to the characteristic blood flow pattern of HCC tumors, a specific perfusion pattern of any detected liver lesion may conclusively detect an HCC tumor. Alternatively, the scan may detect an indeterminate lesion and further evaluation may be performed by obtaining a physical sample of the lesion.[17][38]
Imaging

Ultrasound, CT scan, and MRI may be used to evaluate the liver for HCC. On CT and MRI, HCC can have three distinct patterns of growth:[citation needed]
- A single large tumor
- Multiple tumors
- Poorly defined tumor with an infiltrative growth pattern
A systematic review of CT diagnosis found that the sensitivity was 68% (95% CI 55–80%) and specificity was 93% (95% CI 89–96%) compared with pathologic examination of an explanted or resected liver as the reference standard. With triple-phase helical CT, the sensitivity was 90% or higher, but these data have not been confirmed with autopsy studies.[36]
However, MRI has the advantage of delivering high-resolution images of the liver without ionizing radiation. HCC appears as a high-intensity pattern on T2-weighted images and a low-intensity pattern on T1-weighted images. The advantage of MRI is that it has improved sensitivity and specificity when compared to ultrasound and CT in cirrhotic patients with whom it can be difficult to differentiate HCC from regenerative nodules. A systematic review found that the sensitivity was 81% (95% CI 70–91%) and specificity was 85% (95% CI 77–93%) compared with pathologic examination of an explanted or resected liver as the reference standard.[36] The sensitivity is further increased if gadolinium contrast-enhanced and diffusion-weighted imaging are combined.
MRI is more sensitive and specific than CT.[39]
Liver image reporting and data system (LI-RADS) is a classification system for the reporting of liver lesions detected on CT and MRI. Radiologists use this standardized system to report on suspicious lesions and to provide an estimated likelihood of malignancy. Categories range from LI-RADS (LR) 1 to 5, in order of concern for cancer.[40] A biopsy is not needed to confirm the diagnosis of HCC if certain imaging criteria are met.[17]
Pathology

Macroscopically, liver cancer appears as a nodular or infiltrative tumor. The nodular type may be solitary (large mass) or multiple (when developed as a complication of cirrhosis). Tumor nodules are round to oval, gray or green (if the tumor produces bile), well circumscribed but not encapsulated. The diffuse type is poorly circumscribed and infiltrates the portal veins, or the hepatic veins (rarely).[17]
Microscopically, the four architectural and cytological types (patterns) of hepatocellular carcinoma are: fibrolamellar, pseudoglandular (adenoid), pleomorphic (giant cell), and clear cell. In well-differentiated forms, tumor cells resemble hepatocytes, form trabeculae, cords, and nests, and may contain bile pigment in the cytoplasm. In poorly differentiated forms, malignant epithelial cells are discohesive, pleomorphic, anaplastic, and giant. The tumor has a scant stroma and central necrosis because of the poor vascularization.[41] A fifth form – lymphoepithelioma like hepatocellular carcinoma – has also been described.[42][43]
Staging
BCLC Staging System
The prognosis of HCC is affected by the staging of the tumor and the liver's function due to the effects of liver cirrhosis.[44]
A number of staging classifications for HCC are available. However, due to the unique nature of the carcinoma to fully encompass all the features that affect the categorization of the HCC, a classification system should incorporate tumor size and number, presence of vascular invasion and extrahepatic spread, liver function (levels of serum bilirubin and albumin, presence of ascites, and portal hypertension) and general health status of the patient (defined by the ECOG classification and the presence of symptoms).[44]
Of all the staging classification systems available, the Barcelona Clinic Liver Cancer staging classification encompasses all of the above characteristics. This staging classification can be used to select people for treatment.[45]
| Stage | Description | Child-Pugh class | ECOG performance status |
|---|---|---|---|
| 0 (very early stage) | Single nodule, < 3 cm | A | 0 |
| A (early stage) | 1–3 nodule, all < 3 cm | A or B | |
| B (intermediate stage) | Multi-nodular tumor | ||
| C (advanced stage) | Portal invasion and extra-hepatic spread | 1 or 2 | |
| D (terminal stage) | Severe liver damage | C | 3 or 4 |
Important features that guide treatment include:
- size
- spread (stage)
- involvement of liver vessels
- presence of a tumor capsule
- presence of extrahepatic metastases
- presence of daughter nodules
- vascularity of the tumor
MRI is the best imaging method to detect the presence of a tumor capsule.
The most common sites of metastasis are the lung, abdominal lymph nodes, and bone.[49]
Prevention
Since hepatitis B and C are some of the main causes of hepatocellular carcinoma, prevention of infection is key to then prevent HCC. Thus, childhood vaccination against hepatitis B may reduce the risk of liver cancer in the future.[50] In the case of patients with cirrhosis, alcohol consumption is to be avoided. Also, screening for hemochromatosis may be beneficial for some patients.[51] Whether screening those with chronic liver disease for HCC improves outcomes is unclear.[52]
Treatment
Treatment of hepatocellular carcinoma varies by the stage of disease, a person's likelihood to tolerate surgery, and availability of liver transplant:
- Curative intention: for limited disease, when the cancer is limited to one or more areas of within the liver, surgically removing the malignant cells may be curative. This may be accomplished by resection the affected portion of the liver (partial hepatectomy) or in some cases by orthotopic liver transplantation of the entire organ.[citation needed]
- "Bridging" intention: for limited disease which qualifies for potential liver transplantation, the person may undergo targeted treatment of some or all of the known tumor while waiting for a donor organ to become available.[53]
- "Downstaging" intention: for moderately advanced disease which has not spread beyond the liver, but is too advanced to qualify for curative treatment. The person may be treated by targeted therapies in order to reduce the size or number of active tumors, with the goal of once again qualifying for liver transplant after this treatment.[53]
- Palliative intention: for more advanced disease, including spread of cancer beyond the liver or in persons who may not tolerate surgery, treatment intended to decrease symptoms of disease and maximize duration of survival.[citation needed]
Loco-regional therapy (also referred to as liver-directed therapy) refers to any one of several minimally-invasive treatment techniques to focally target HCC within the liver. These procedures are alternatives to surgery, and may be considered in combination with other strategies, such as a later liver transplantation.[54] Generally, these treatment procedures are performed by interventional radiologists or surgeons, in coordination with a medical oncologist. Loco-regional therapy may refer to either percutaneous therapies (e.g. cryoablation), or arterial catheter-based therapies (chemoembolization or radioembolization).[citation needed]
Surgical resection
Surgical removal of the tumor is associated with better cancer prognosis, but only 5–15% of patients are suitable for surgical resection due to the extent of disease or poor liver function.[55] Surgery is only considered if the entire tumor can be safely removed while preserving sufficient functional liver to maintain normal physiology. Thus, preoperative imaging assessment is critical to determine both the extent of HCC and to estimate the amount of residual liver remaining after surgery. To maintain liver function, residual liver volume should exceed 25% of total liver volume in a noncirrhotic liver, greater than 40% in a cirrhotic liver.[56] Surgery on diseased or cirrhotic livers is generally associated with higher morbidity and mortality. The overall recurrence rate after resection is 50–60%. The Singapore Liver Cancer Recurrence score can be used to estimate risk of recurrence after surgery.[57]
Liver transplantation
Liver transplantation, replacing the diseased liver with a cadaveric or a living donor liver, plays an increasing role in treatment of HCC. Although outcomes following liver transplant were initially poor (20%–36% survival rate),[17] outcomes have significantly improved with improvement in surgical techniques and adoption of the Milan criteria at US transplantation centers. Expanded Shanghai criteria in China have resulted in overall survival and disease-free survival rates similar to those achieved using the Milan criteria.[58] Studies from the late 2000s obtained higher survival rates ranging from 67% to 91%.[59]
The risks of liver transplantation extend beyond risk of the procedure itself. The immunosuppressive medication required after surgery to prevent rejection of the donor liver also impairs the body's natural ability to combat dysfunctional cells. If the tumor has spread undetected outside the liver before the transplant, the medication effectively increases the rate of disease progression and decreases survival. With this in mind, liver transplant "can be a curative approach for patients with advanced HCC without extrahepatic metastasis".[60] In fact, among patients with compensated cirrhosis, transplantation is not associated with improved survival compared to hepatectomy, but instead is significantly more expensive.[61] Patient selection is considered a major key for success.[62]
Ablation
- Radiofrequency ablation (RFA) uses high-frequency radio waves to destroy tumor by local heating. The electrodes are inserted into the liver tumor under ultrasound image guidance using percutaneous, laparoscopic or open surgical approach. It is suitable for small tumors (<5 cm). RFA has the best outcomes in patients with a solitary tumor less than 4 cm.[63] Since it is a local treatment and has minimal effect on normal healthy tissue, it can be repeated multiple times. Survival is better for those with smaller tumors. In one study, In one series of 302 patients, the three-year survival rates for lesions >5 cm, 2.1 to 5 cm, and ≤2 cm were 59, 74, and 91%, respectively.[64] A large randomized trial comparing surgical resection and RFA for small HCC showed similar four-year survival and less morbidities for patients treated with RFA.[65]
- Cryoablation is a technique used to destroy tissue using cold temperature. The tumor is not removed and the destroyed cancer is left to be reabsorbed by the body. Initial results in properly selected patients with unresectable liver tumors are equivalent to those of resection. Cryosurgery involves the placement of a stainless steel probe into the center of the tumor. Liquid nitrogen is circulated through the end of this device. The tumor and a half inch margin of normal liver are frozen to −190 °C for 15 minutes, which is lethal to all tissues. The area is thawed for 10 minutes and then refrozen to −190 °C for another 15 minutes. After the tumor has thawed, the probe is removed, bleeding is controlled, and the procedure is complete. The patient spends the first postoperative night in the intensive care unit and typically is discharged in 3–5 days. Proper selection of patients and attention to detail in performing the cryosurgical procedure are mandatory to achieve good results and outcomes. Frequently, cryosurgery is used in conjunction with liver resection, as some of the tumors are removed while others are treated with cryosurgery.[citation needed]
- Percutaneous ethanol injection is well tolerated, with high RR in small (<3 cm) solitary tumors; as of 2005, no randomized trial has comparing resection to percutaneous treatments; recurrence rates are similar to those for postresection. However, a comparative study found that local therapy can achieve a 5-year survival rate around 60% for patients with small HCC.[66]
Arterial catheter-based treatment
- Transcatheter arterial chemoembolization (TACE) is performed for unresectable tumors or as a temporary treatment while waiting for liver transplant ("bridge to transplant"). TACE is done by injecting an antineoplastic drug (e.g. cisplatin) mixed with a radio-opaque contrast (e.g. Lipiodol) and an embolic agent (e.g. Gelfoam) into the right or left hepatic artery via the groin artery. The goal of the procedure is to restrict the tumor's vascular supply while supplying a targeted chemotherapeutic agent. TACE has been shown to increase survival and to downstage HCC in patients who exceed the Milan criteria for liver transplant. Patients who undergo the procedure are followed with CT scans and may need additional TACE procedures if the tumor persists.[67] As of 2005, multiple trials show objective tumor responses and slowed tumor progression, but questionable survival benefit compared to supportive care; greatest benefit is seen in people with preserved liver function, absence of vascular invasion, and smallest tumors. TACE is not suitable for big tumors (>8 cm), the presence of portal vein thrombus, tumors with a portal-systemic shunt, and patients with poor liver function.[citation needed]
- Selective internal radiation therapy (SIRT) can be used to destroy the tumor from within (thus minimizing exposure to healthy tissue). Similar to TACE, this is a procedure in which an interventional radiologist selectively injects the artery or arteries supplying the tumor with a chemotherapeutic agent. The agent is typically Yttrium-90 (Y-90) incorporated into embolic microspheres that lodge in the tumor vasculature, causing ischemia and delivering their radiation dose directly to the lesion. This technique allows for a higher, local dose of radiation to be delivered directly to the tumor while sparing normal healthy tissue. While not curative, patients have increased survival. No studies have been done to compare whether SIRT is superior to TACE in terms of survival outcomes, although retrospective studies suggest similar efficacy.[68] Two products are available, SIR-Spheres and TheraSphere. The latter is an FDA-approved treatment for primary liver cancer (HCC) which has been shown in clinical trials to increase the survival rate of low-risk patients. SIR-Spheres are FDA-approved for the treatment of metastatic colorectal cancer, but outside the US, SIR-Spheres are approved for the treatment of any nonresectable liver cancer including primary liver cancer.[69]
External beam therapy
- The role of radiotherapy in the treatment of hepatocellular carcinoma has evolved as technological advancements in treatment delivery and imaging have provided a means for safe and effective radiotherapy delivery in a wide spectrum of HCC patients. In metastatic cases, radiotherapy can be used for palliative care.[70][71]
- Proton therapy for unresectable hepatocellular carcinoma was associated with improved survival relative to photon-based radiation therapy which may be driven by decreased incidence of post-treatment liver decompensation[72] and a number of randomized controlled trials are currently ongoing.[73][74][75]
Systemic
In disease which has spread beyond the liver, systemic therapy may be a consideration. In 2007, Sorafenib, an oral multikinase inhibitor, was the first systemic agent approved for first-line treatment of advanced HCC.[76] Trials have found modest improvement in overall survival: 10.7 months vs 7.9 months and 6.5 months vs 4.2 months.[77][76]
The most common side effects of Sorafenib include a hand-foot skin reaction and diarrhea.[77] Sorafenib is thought to work by blocking growth of both tumor cells and new blood vessels. Numerous other molecular targeted drugs are being tested as alternative first- and second-line treatments for advanced HCC.[78]
A host of additional targeted therapies and immune checkpoint inhibitors have been found to be effective against this disease. For instance, in the recent phase III trial IMBrave 150, the combination of atezolizumab and bevacizumab was found to improve both overall and progression-free survival compared to sorafenib alone.[79]
Tremelimumab (Imjudo) was approved for medical use in the United States in October 2022.[80] It is indicated, in combination with durvalumab, for the treatment of adults with unresectable hepatocellular carcinoma.[80]
Other
- Portal vein embolization (PVE): This technique is sometimes used to increase the volume of healthy liver, in order to improve chances of survival following surgical removal of diseased liver. For example, embolization of the right main portal vein would result in compensatory hypertrophy of the left lobe, which may qualify the patient for a partial hepatectomy. Embolization is performed by an interventional radiologist using a percutaneous transhepatic approach. This procedure can also serve as a bridge to transplant.[81]
- High intensity focused ultrasound (HIFU) (as opposed to diagnostic ultrasound) is an experimental technique which uses high-powered ultrasound waves to destroy tumor tissue.
- A systematic review assessed 12 articles involving a total of 318 patients with hepatocellular carcinoma treated with Yttrium-90 radioembolization.[82] Excluding a study of only one patient, post-treatment CT evaluation of the tumor showed a response ranging from 29 to 100% of patients evaluated, with all but two studies showing a response of 71% or greater.
Prognosis
The usual outcome is poor because only 10–20% of hepatocellular carcinomas can be removed completely using surgery. If the cancer cannot be completely removed, the disease is usually deadly within 3 to 6 months.[83] [failed verification] This is partially due to late presentation with tumors, but also the lack of medical expertise and facilities in the regions with high HCC prevalence. However, survival can vary, and occasionally people survive much longer than 6 months. The prognosis for metastatic or unresectable HCC has improved due to the approval of Sorafenib (Nexavar®) for advanced HCC.[citation needed]
Epidemiology

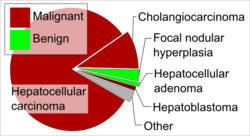
HCC is one of the most common tumors worldwide. The epidemiology of HCC exhibits two main patterns, one in North America and Western Europe and another in non-Western countries, such as those in sub-Saharan Africa, Central and Southeast Asia, and the Amazon basin. Males are affected more than females usually, and it is most common between the ages of 30 and 50,[5]:821–881 Hepatocellular carcinoma causes 662,000 deaths worldwide per year[86] about half of them in China.
Africa and Asia
In some parts of the world, such as sub-Saharan Africa and Southeast Asia, HCC is the most common cancer, generally affecting men more than women, and with an age of onset between the late teens and 30s.[17] This variability is in part due to the different patterns of hepatitis B and hepatitis C transmission in different populations – infection at or around birth predispose to earlier cancers than if people are infected later. The time between hepatitis B infection and development into HCC can be years, even decades, but from diagnosis of HCC to death, the average survival period is only 5.9 months according to one Chinese study during the 1970-80s, or 3 months (median survival time) in sub-Saharan Africa according to Manson's textbook of tropical diseases. HCC is one of the deadliest cancers in China, where chronic hepatitis B is found in 90% of cases. In Japan , chronic hepatitis C is associated with 90% of HCC cases. Foods infected with Aspergillus flavus (especially peanuts and corns stored during prolonged wet seasons) which produces aflatoxins pose another risk factor for HCC.[87]
North America and Western Europe
The most common malignant tumors in the liver represent metastases (spread) from tumors which originate elsewhere in the body.[5] Among cancers that originate from liver tissue, HCC is the most common primary liver cancer. In the United States, the US surveillance, epidemiology, and end results database program, shows that HCC accounts for 65% of all cases of liver cancers.[88] As screening programs are in place for high-risk persons with chronic liver disease, HCC is often discovered much earlier in Western countries than in developing regions such as sub-Saharan Africa.[citation needed]
Acute and chronic hepatic porphyrias (acute intermittent porphyria, porphyria cutanea tarda, hereditary coproporphyria, variegate porphyria) and tyrosinemia type I are risk factors for hepatocellular carcinoma. The diagnosis of an acute hepatic porphyria (AIP, HCP, VP) should be sought in patients with HCC without typical risk factors of hepatitis B or C, alcoholic liver cirrhosis, or hemochromatosis. Both active and latent genetic carriers of acute hepatic porphyrias are at risk for this cancer, although latent genetic carriers have developed the cancer at a later age than those with classic symptoms. Patients with acute hepatic porphyrias should be monitored for HCC.[citation needed]
The incidence of HCC is relatively lower in the Western Hemisphere than in Eastern Asia. However, despite the statistics being low, the diagnosis of HCC has increased since the 1980s and it is continuing to increase, making it one of the rising causes of death due to cancer. The common risk factor for HCC is hepatitis C, along with other health issues.[89][90]
Research
Preclinical
Mipsagargin (G-202), has orphan drug designation as a treatment during chemotherapy for HCC.[91] It is a thapsigargin-based prodrug with cytotoxic activity used to reduce blood flow to the tumor during treatment. Results from Phase 2 trial recommended G-202 as a first-in-class PSMA-targeted prodrug and that it move to clinical trials.[92]
Current research includes the search for the genes that are disregulated in HCC, antiheparanase antibodies,[93] protein markers,[94] non-coding RNAs[95] (such as TUC338)[96] and other predictive biomarkers.[97][98] As similar research is yielding results in various other malignant diseases, it is hoped that identifying the aberrant genes and the resultant proteins could lead to the identification of pharmacological interventions for HCC.[99]
The development of three-dimensional culture methods provides a new approach for preclinical studies of cancer therapy using patient-derived organoids. These miniaturized organoid 'avatars' of a patient's tumor recapitulate several features of the original tumor, rendering them an attractive model for drug-sensitivity testing and precision medicine for HCC and other types of primary liver cancer.[100]
Furthermore, HCC occurs in patients with liver disease. A biomarker named six-miRNA signature allows effective treatment of patients with HCC and is able to predict its recurrence in the liver.[101]
A prospective study found that increased hepatocellular cancer risk is associated with higher levels of major circulating bile acids that were measured in people several years prior to tumor diagnosis.[102] In another study using a mouse model, it was found that dysregulated hepatic bile acids collaboratively promote liver carcinogenesis.[103]
Clinical
JX-594, an oncolytic virus, has orphan drug designation for this condition and is undergoing clinical trials.[104] Hepcortespenlisimut-L (Hepko-V5), an oral cancer vaccine, also has US FDA orphan drug designation for HCC.[105] Immunitor Inc. completed a Phase II trial, published in 2017.[106] A randomized trial of people with advanced HCC showed no benefit for the combination of everolimus and pasireotide.[107]
See also
- Hemihypertrophy
- Oncovirus
- Portal hypertension
References
- ↑ 1.0 1.1 "Epidemiology of hepatocellular carcinoma (HCC) in hemophilia". Critical Reviews in Oncology/Hematology 99: 129–133. March 2016. doi:10.1016/j.critrevonc.2015.12.009. PMID 26754251.
- ↑ "Hepatocellular carcinoma". Lancet 379 (9822): 1245–1255. March 2012. doi:10.1016/S0140-6736(11)61347-0. PMID 22353262.
- ↑ "Global Cancer Observatory". http://gco.iarc.fr/.
- ↑ "A Meta-Analysis on the Rate of Hepatocellular Carcinoma Recurrence after Liver Transplant and Associations to Etiology, Alpha-Fetoprotein, Income and Ethnicity". Journal of Clinical Medicine 10 (2): 238. January 2021. doi:10.3390/jcm10020238. PMID 33440759.
- ↑ 5.0 5.1 5.2 5.3 Robbins & Cotran Pathologic Basis of Disease (9th ed.). Saunders. 2015. ISBN 978-1-4557-2613-4.
- ↑ 6.0 6.1 "Liver cancer overview". Mayo Clinic. http://www.mayoclinic.com/health/liver-cancer/DS00399/DSECTION=symptoms.
- ↑ 7.0 7.1 "Cirrhosis and chronic liver failure: part I. Diagnosis and evaluation". American Family Physician 74 (5): 756–762. September 2006. PMID 16970019.
- ↑ "Epidemiology of hepatitis C virus infection". World Journal of Gastroenterology 13 (17): 2436–2441. May 2007. doi:10.3748/wjg.v13.i17.2436. PMID 17552026.
- ↑ "Association between nonalcoholic fatty liver disease and risk for hepatocellular cancer, based on systematic review". Clinical Gastroenterology and Hepatology 10 (12): 1342–1359.e2. December 2012. doi:10.1016/j.cgh.2012.10.001. PMID 23041539.
- ↑ "NAFLD vs. NASH" (in en-US). 2019-11-11. https://medicinespecifics.com/nafld-vs-nash/.
- ↑ 11.0 11.1 11.2 "The association between diabetes and hepatocellular carcinoma: a systematic review of epidemiologic evidence". Clinical Gastroenterology and Hepatology 4 (3): 369–380. March 2006. doi:10.1016/j.cgh.2005.12.007. PMID 16527702. "Diabetes is associated with an increased risk for HCC. However, more research is required to examine issues related to the duration and treatment of diabetes, and confounding by diet and obesity".
- ↑ "Molecular pathogenesis of human hepatocellular carcinoma". Toxicology 181-182: 43–47. December 2002. doi:10.1016/S0300-483X(02)00253-6. PMID 12505283. https://zenodo.org/record/1259987. "Recent studies in our laboratory have identified several potential factors that may contribute to the pathogenesis of HCC...For example, oxyradical overload diseases such as Wilson disease and hemochromatosis result in the generation of oxygen/nitrogen species that can cause mutations in the p53 tumour suppressor gene".
- ↑ "Hepatocellular carcinoma in a case of Wilson's disease". Liver 12 (1): 42–45. February 1992. doi:10.1111/j.1600-0676.1992.tb00553.x. PMID 1314321. "The patient described here was the oldest and only the third female patient with hepatocellular carcinoma complicating Wilson's disease to be reported in the literature".
- ↑ "Wilson's disease and hepatocellular carcinoma: possible protective role of copper". Gut 24 (8): 767–771. August 1983. doi:10.1136/gut.24.8.767. PMID 6307837. "As copper has been shown to protect against chemically induced hepatocellular carcinoma in rats, this may be the reason for the extreme rarity of hepatocellular carcinoma in patients with Wilson's disease and possibly in other liver diseases with hepatic copper overload".
- ↑ "Incidence and survival of cancers among 1,054 hemophilia patients: A nationwide and 14-year cohort study". American Journal of Hematology 90 (4): E55–E59. April 2015. doi:10.1002/ajh.23947. PMID 25639564.
- ↑ "Hepatitis B and C virus infection and hepatocellular carcinoma in China: a review of epidemiology and control measures". Journal of Epidemiology 21 (6): 401–416. 2011. doi:10.2188/jea.JE20100190. PMID 22041528.
- ↑ 17.0 17.1 17.2 17.3 17.4 17.5 17.6 17.7 17.8 17.9 "Hepatocellular carcinoma: a review". Journal of Hepatocellular Carcinoma 3: 41–53. 2016. doi:10.2147/JHC.S61146. PMID 27785449.
- ↑ "Pathophysiology". Emedicine. MedScape. 2019-11-10. http://emedicine.medscape.com/article/986988-overview.
- ↑ 19.0 19.1 19.2 "Association of diabetes duration and diabetes treatment with the risk of hepatocellular carcinoma". Cancer 116 (8): 1938–1946. April 2010. doi:10.1002/cncr.24982. PMID 20166205. "Diabetes appears to increase the risk of HCC, and such risk is correlated with a long duration of diabetes. Relying on dietary control and treatment with sulfonylureas or insulin were found to confer the highest magnitude of HCC risk, whereas treatment with biguanides or thiazolidinediones was associated with a 70% HCC risk reduction among diabetics.".
- ↑ Charitha, Gorantla Sri; Chaitanya, Nyshadham S. N.; Reddy, Aramati Bindu Madhava (2022-01-01), Nagaraju, Ganji Purnachandra; Vadde, Ramakrishna, eds., "Chapter 22 - LKB1/STK11-mediated signal transduction in hepatocellular carcinoma" (in en), Theranostics and Precision Medicine for the Management of Hepatocellular Carcinoma, Volume 2 (Academic Press): pp. 357–367, doi:10.1016/b978-0-323-98807-0.00017-x, ISBN 978-0-323-98807-0, https://www.sciencedirect.com/science/article/pii/B978032398807000017X, retrieved 2023-04-06
- ↑ 21.0 21.1 "Antidiabetic therapy and increased risk of hepatocellular carcinoma in chronic liver disease". World Journal of Gastroenterology 15 (20): 2506–2511. May 2009. doi:10.3748/wjg.15.2506. PMID 19469001. "Our study confirms that type 2 diabetes mellitus is an independent risk factor for HCC and pre-exists in the majority of HCC patients. Moreover, in male patients with type 2 diabetes mellitus, our data shows a direct association of HCC with insulin and sulphanylureas treatment and an inverse relationship with metformin therapy.".
- ↑ "Antidiabetic therapy and increased risk of hepatocellular carcinoma in chronic liver disease". World Journal of Gastroenterology 15 (20): 2506–2511. May 2009. doi:10.3748/wjg.15.2506. PMID 19469001.
- ↑ "Metabolic syndrome and hepatocellular carcinoma: two growing epidemics with a potential link". Cancer 115 (24): 5651–5661. December 2009. doi:10.1002/cncr.24687. PMID 19834957. "The majority of 'cryptogenic' HCC in the United States is attributed to nonalcoholic fatty liver disease (NAFLD), a hepatic manifestation of the metabolic syndrome... It is predicted that metabolic syndrome will lead to large increases in the incidence of HCC over the next decades. A better understanding of the relation between these two diseases ultimately should lead to improved screening and treatment options for patients with HCC.".
- ↑ "Non-alcoholic fatty liver disease as a risk factor for hepatocellular carcinoma: mechanisms and implications". Gut 59 (10): 1303–1307. October 2010. doi:10.1136/gut.2009.199661. PMID 20650925. "Based on the known association of NAFLD with IR and MS, approximately two-thirds of the patients were obese and/or diabetic, 4 and a remarkable 25% of these patients had no cirrhosis... Therefore, it is particularly worrying that the most persuasive evidence for an association between NAFLD and HCC derives from studies on the risk of HCC in patients with metabolic syndrome".
- ↑ "Hepatocellular Carcinoma and Diseases". http://www.hepatocellular.org/.
- ↑ "Blockade of IGF-1 receptor tyrosine kinase has antineoplastic effects in hepatocellular carcinoma cells". Biochemical Pharmacology 71 (10): 1435–1448. May 2006. doi:10.1016/j.bcp.2006.02.006. PMID 16530734. "Inhibition of IGF-1R tyrosine kinase (IGF-1R-TK) by NVP-AEW541 induces growth inhibition, apoptosis and cell cycle arrest in human HCC cell lines without accompanying cytotoxicity. Thus, IGF-1R-TK inhibition may be a promising novel treatment approach in HCC.".
- ↑ "A possible role for insulin-like growth factor-binding protein-3 autocrine/paracrine loops in controlling hepatocellular carcinoma cell proliferation". Cell Growth & Differentiation 13 (3): 115–122. March 2002. PMID 11959812. http://cgd.aacrjournals.org/cgi/reprint/13/3/115. "Our data indicate that loss of autocrine/paracrine IGFBP-3 loops may lead to HCC tumor growth and suggest that modulating production of the IGFs, IGFBP-3, and IGF-IR may represent a novel approach in the treatment of HCC.".
- ↑ "Anabolic steroid abuse causing recurrent hepatic adenomas and hemorrhage". World Journal of Gastroenterology 14 (28): 4573–4575. July 2008. doi:10.3748/wjg.14.4573. PMID 18680242. "This is the first reported case of hepatic adenoma re-growth with recidivistic steroid abuse, complicated by life-threatening hemorrhage.".
- ↑ "Hepatocellular carcinoma associated with recreational anabolic steroid use". British Journal of Sports Medicine 42 (1): 74–5; discussion 75. January 2008. doi:10.1136/bjsm.2007.03932. PMID 18178686. "Malignant transformation to HCC from a pre-existing hepatic adenoma confirmed by immunohistochemical study has previously not been reported in athletes taking anabolic steroids. Further studies using screening programmes to identify high-risk individuals are recommended.".
- ↑ "Exploration of liver cancer genomes". Nature Reviews. Gastroenterology & Hepatology 11 (6): 340–349. June 2014. doi:10.1038/nrgastro.2014.6. PMID 24473361.
- ↑ "Risk of hepatocellular carcinoma across a biological gradient of serum hepatitis B virus DNA level". JAMA 295 (1): 65–73. January 2006. doi:10.1001/jama.295.1.65. PMID 16391218.
- ↑ "Involvement of DNA damage response pathways in hepatocellular carcinoma". BioMed Research International 2014: 153867. 2014. doi:10.1155/2014/153867. PMID 24877058.
- ↑ "Oxidative stress and epigenetic instability in human hepatocarcinogenesis". Digestive Diseases 31 (5–6): 447–453. 2013. doi:10.1159/000355243. PMID 24281019.
- ↑ 34.0 34.1 "AASLD guidelines for the treatment of hepatocellular carcinoma". Hepatology 67 (1): 358–380. January 2018. doi:10.1002/hep.29086. PMID 28130846.
- ↑ "Clinical features and diagnosis of primary hepatocellular carcinoma". UptoDate. http://www.uptodate.com/contents/clinical-features-and-diagnosis-of-primary-hepatocellular-carcinoma?source=see_link.
- ↑ 36.0 36.1 36.2 "Accuracy of ultrasonography, spiral CT, magnetic resonance, and alpha-fetoprotein in diagnosing hepatocellular carcinoma: a systematic review". The American Journal of Gastroenterology 101 (3): 513–523. March 2006. doi:10.1111/j.1572-0241.2006.00467.x. PMID 16542288.
- ↑ "A combination of α-fetoprotein and des-γ-carboxy prothrombin is superior in detection of hepatocellular carcinoma". Digestion 87 (2): 121–131. 2013. doi:10.1159/000346080. PMID 23406785.
- ↑ "Benign Liver Tumors". https://www.lecturio.com/concepts/benign-liver-tumors/.
- ↑ "Diagnosis and treatment of hepatocellular carcinoma". Gastroenterology 134 (6): 1752–1763. May 2008. doi:10.1053/j.gastro.2008.02.090. PMID 18471552.
- ↑ "Li-Rads". http://www.acr.org/Quality-Safety/Resources/LIRADS.
- ↑ Hepatocellular carcinoma (Photo) ATLAS OF PATHOLOGY
- ↑ "Genomic landscape of lymphoepithelioma-like hepatocellular carcinoma". The Journal of Pathology 249 (2): 166–172. October 2019. doi:10.1002/path.5313. PMID 31168847.
- ↑ "Lymphoepithelioma-like hepatocellular carcinoma: an uncommon variant of hepatocellular carcinoma with favorable outcome". The American Journal of Surgical Pathology 39 (3): 304–312. March 2015. doi:10.1097/pas.0000000000000376. PMID 25675010.
- ↑ 44.0 44.1 "Staging of hepatocellular carcinoma". Journal of Clinical and Experimental Hepatology 4 (Suppl 3): S74–S79. August 2014. doi:10.1016/j.jceh.2014.03.045. PMID 25755615.
- ↑ "Prognosis of hepatocellular carcinoma: the BCLC staging classification". Seminars in Liver Disease 19 (3): 329–338. 1999. doi:10.1055/s-2007-1007122. PMID 10518312.
- ↑ "BCLC staging system and the Child-Pugh system ;Liver cancer ; Cancer Research UK". https://www.cancerresearchuk.org/about-cancer/liver-cancer/stages/bclc-staging-system-child-pugh-system.
- ↑ "What is the Barcelona Clinic Liver Cancer (BCLC) system for hepatocellular carcinoma (HCC) staging?". https://www.medscape.com/answers/197319-39221/what-is-the-barcelona-clinic-liver-cancer-bclc-system-for-hepatocellular-carcinoma-hcc-staging.
- ↑ "Staging systems for hepatocellular carcinoma: Current status and future perspectives". World Journal of Hepatology 7 (3): 406–424. March 2015. doi:10.4254/wjh.v7.i3.406. PMID 25848467.
- ↑ "Extrahepatic metastases of hepatocellular carcinoma". Radiology 216 (3): 698–703. September 2000. doi:10.1148/radiology.216.3.r00se24698. PMID 10966697.
- ↑ "Hepatitis B: Prevention and treatment". https://www.who.int/csr/disease/hepatitis/whocdscsrlyo20022/en/index5.html. "WHO aims at controlling HBV worldwide to decrease the incidence of HBV-related chronic liver disease, cirrhosis, and hepatocellular carcinoma. by integrating HB vaccination into routine infant (and possibly adolescent) immunization programs."
- ↑ "Prevention". https://www.nlm.nih.gov/medlineplus/ency/article/000280.htm.
- ↑ "Screening for hepatocellular carcinoma in chronic liver disease: a systematic review". Annals of Internal Medicine 161 (4): 261–269. August 2014. doi:10.7326/M14-0558. PMID 24934699.
- ↑ 53.0 53.1 "Bridging and downstaging treatments for hepatocellular carcinoma in patients on the waiting list for liver transplantation". World Journal of Gastroenterology 19 (43): 7515–7530. November 2013. doi:10.3748/wjg.v19.i43.7515. PMID 24282343.
- ↑ "Locoregional and systemic therapy for hepatocellular carcinoma". Journal of Gastrointestinal Oncology 8 (2): 215–228. April 2017. doi:10.21037/jgo.2017.03.13. PMID 28480062.
- ↑ "Diagnosis, Staging, and Management of Hepatocellular Carcinoma: 2018 Practice Guidance by the American Association for the Study of Liver Diseases". Hepatology 68 (2): 723–750. August 2018. doi:10.1002/hep.29913. PMID 29624699.[yes|permanent dead link|dead link}}]
- ↑ "Surgical resection of localized hepatocellular carcinoma: patient selection and special consideration". Journal of Hepatocellular Carcinoma 4: 1–9. December 2016. doi:10.2147/JHC.S96085. PMID 28097107.
- ↑ "The Singapore Liver Cancer Recurrence (SLICER) Score for relapse prediction in patients with surgically resected hepatocellular carcinoma". PLOS ONE 10 (4): e0118658. 2015. doi:10.1371/journal.pone.0118658. PMID 25830231. Bibcode: 2015PLoSO..1018658A.
- ↑ "Liver transplantation outcomes in 1,078 hepatocellular carcinoma patients: a multi-center experience in Shanghai, China". Journal of Cancer Research and Clinical Oncology 135 (10): 1403–1412. October 2009. doi:10.1007/s00432-009-0584-6. PMID 19381688.
- ↑ "Long-term results of liver transplantation for hepatocellular carcinoma: an update of the University of Padova experience". Transplantation Proceedings 39 (6): 1892–1894. 2007. doi:10.1016/j.transproceed.2007.05.031. PMID 17692645.
- ↑ "Liver transplantation as curative approach for advanced hepatocellular carcinoma: is it justified?". Langenbeck's Archives of Surgery 393 (2): 141–147. March 2008. doi:10.1007/s00423-007-0250-x. PMID 18043937.
- ↑ "Hepatocellular Carcinoma in Transplantable Child-Pugh A Cirrhotics: Should Cost Affect Resection vs Transplantation?". Journal of Gastrointestinal Surgery 23 (6): 1135–1142. June 2019. doi:10.1007/s11605-018-3946-z. PMID 30218342.
- ↑ "Liver transplantation for the treatment of moderately or well-differentiated hepatocellular carcinoma". Annals of Surgery 239 (2): 150–159. February 2004. doi:10.1097/01.sla.0000109146.72827.76. PMID 14745321.
- ↑ "Radiofrequency ablation: the experts weigh in". Cancer 100 (3): 641–650. February 2004. doi:10.1002/cncr.11919. PMID 14745883.
- ↑ "Percutaneous radiofrequency ablation for hepatocellular carcinoma. An analysis of 1000 cases". Cancer 103 (6): 1201–1209. March 2005. doi:10.1002/cncr.20892. PMID 15690326.
- ↑ "A prospective randomized trial comparing percutaneous local ablative therapy and partial hepatectomy for small hepatocellular carcinoma". Annals of Surgery 243 (3): 321–328. March 2006. doi:10.1097/01.sla.0000201480.65519.b8. PMID 16495695.
- ↑ "Treatment strategy for small hepatocellular carcinoma: comparison of long-term results after percutaneous ethanol injection therapy and surgical resection". Hepatology 34 (4 Pt 1): 707–713. October 2001. doi:10.1053/jhep.2001.27950. PMID 11584366.
- ↑ "Interventional Radiology Treatments for Liver Cancer". Society of Interventional Radiology. http://www.sirweb.org/patients/liver-cancer/..
- ↑ "Comparison of yttrium-90 radioembolization and transcatheter arterial chemoembolization for the treatment of unresectable hepatocellular carcinoma". Journal of Vascular and Interventional Radiology 21 (2): 224–230. February 2010. doi:10.1016/j.jvir.2009.10.013. PMID 20022765.
- ↑ "Hepatocellular Carcinoma (HCC) and Liver Metastases". https://www.lecturio.com/concepts/hepatocellular-carcinoma-hcc-and-liver-metastases/.
- ↑ "Hepatocellular carcinoma radiation therapy: review of evidence and future opportunities". International Journal of Radiation Oncology, Biology, Physics 87 (1): 22–32. September 2013. doi:10.1016/j.ijrobp.2012.08.043. PMID 23219567.
- ↑ "Rethinking the Role of Radiation Therapy in the Treatment of Unresectable Hepatocellular Carcinoma: A Data Driven Treatment Algorithm for Optimizing Outcomes". Frontiers in Oncology 9: 345. 2019. doi:10.3389/fonc.2019.00345. PMID 31275846.
- ↑ "Protons versus Photons for Unresectable Hepatocellular Carcinoma: Liver Decompensation and Overall Survival". International Journal of Radiation Oncology, Biology, Physics 105 (1): 64–72. September 2019. doi:10.1016/j.ijrobp.2019.01.076. PMID 30684667.
- ↑ Radiation Therapy With Protons or Photons in Treating Patients With Liver Cancer. 21 Aug 2020. https://clinicaltrials.gov/ct2/show/NCT03186898?term=03186898&rank=1.
- ↑ Proton Radiotherapy Versus Radiofrequency Ablation for Patients With Medium or Large Hepatocellular Carcinoma. 21 Aug 2020. https://clinicaltrials.gov/ct2/show/NCT02640924.
- ↑ Transarterial Chemoembolization Versus Proton Beam Radiotherapy for the Treatment of Hepatocellular Carcinoma. 21 Aug 2020. https://clinicaltrials.gov/ct2/show/NCT00857805.
- ↑ 76.0 76.1 "Sorafenib in advanced hepatocellular carcinoma". The New England Journal of Medicine 359 (4): 378–390. July 2008. doi:10.1056/nejmoa0708857. PMID 18650514.
- ↑ 77.0 77.1 "Efficacy and safety of sorafenib in patients in the Asia-Pacific region with advanced hepatocellular carcinoma: a phase III randomised, double-blind, placebo-controlled trial". The Lancet. Oncology 10 (1): 25–34. January 2009. doi:10.1016/S1470-2045(08)70285-7. PMID 19095497.
- ↑ "Systemic Therapy for Hepatocellular Carcinoma: 2017 Update". Oncology 93 (1): 135–146. 2017. doi:10.1159/000481244. PMID 29258077.
- ↑ "Atezolizumab plus Bevacizumab in Unresectable Hepatocellular Carcinoma". The New England Journal of Medicine 382 (20): 1894–1905. May 2020. doi:10.1056/nejmoa1915745. PMID 32402160.
- ↑ 80.0 80.1 "Imjudo (tremelimumab) in combination with Imfinzi approved in the US for patients with unresectable liver cancer". AstraZeneca (Press release). 26 October 2022. Retrieved 26 October 2022.
- ↑ "Transhepatic portal vein embolization: anatomy, indications, and technical considerations". Radiographics 22 (5): 1063–1076. September–October 2002. doi:10.1148/radiographics.22.5.g02se161063. PMID 12235336.
- ↑ "Yttrium-90 microsphere radioembolization for the treatment of liver malignancies: a structured meta-analysis". European Radiology 19 (4): 951–959. April 2009. doi:10.1007/s00330-008-1211-7. PMID 18989675.
- ↑ Hepatocellular carcinoma MedlinePlus, Medical Encyclopedia
- ↑ "WHO Disease and injury country estimates". World Health Organization. 2009. https://www.who.int/healthinfo/global_burden_disease/estimates_country/en/index.html.
- ↑ Table 37.2 in: Sternberg's diagnostic surgical pathology. Place of publication not identified: LWW. 2012. ISBN 978-1-4511-5289-0. OCLC 953861627.
- ↑ "Cancer". World Health Organization. February 2006. https://www.who.int/mediacentre/factsheets/fs297/en/.
- ↑ "Aflatoxins - Cancer-Causing Substances - NCI" (in en). 2015-03-20. https://www.cancer.gov/about-cancer/causes-prevention/risk/substances/aflatoxins.
- ↑ "Review of hepatocellular carcinoma: Epidemiology, etiology, and carcinogenesis". Journal of Carcinogenesis 16 (1): 1. 2017-01-01. doi:10.4103/jcar.jcar_9_16. PMID 28694740.
- ↑ "Comparison of hepatocellular carcinoma in Eastern versus Western populations". Cancer 122 (22): 3430–3446. November 2016. doi:10.1002/cncr.30237. PMID 27622302.
- ↑ "Changing epidemiology of hepatocellular carcinoma in Asia". Best Practice & Research. Clinical Gastroenterology 29 (6): 919–928. December 2015. doi:10.1016/j.bpg.2015.09.007. PMID 26651253.
- ↑ phyton (2016-08-22). "Phyton Biotech Achieves Manufacturing Milestone with Thapsigargin, the Active Agent in Mipsagargin" (in en-US). https://phytonbiotech.com/phyton-biotech-achieves-manufacturing-milestone-with-thapsigargin-the-active-agent-in-mipsagargin/.
- ↑ "A Phase II, Multicenter, Single-Arm Study of Mipsagargin (G-202) as a Second-Line Therapy Following Sorafenib for Adult Patients with Progressive Advanced Hepatocellular Carcinoma". Cancers 11 (6): 833. June 2019. doi:10.3390/cancers11060833. PMID 31212948.
- ↑ "Screening and identification of novel B cell epitopes in human heparanase and their anti-invasion property for hepatocellular carcinoma". Cancer Immunology, Immunotherapy 58 (9): 1387–1396. September 2009. doi:10.1007/s00262-008-0651-x. PMID 19169879.
- ↑ "Huntington Medical Research Institute News, May 2005". http://www.hmri.org/HMRI_News/Resources/newsletter_May_05.pdf.
- ↑ "Non-coding RNA in hepatocellular carcinoma: Mechanisms, biomarkers and therapeutic targets". Journal of Hepatology 67 (3): 603–618. September 2017. doi:10.1016/j.jhep.2017.04.009. PMID 28438689.
- ↑ "Expression and functional role of a transcribed noncoding RNA with an ultraconserved element in hepatocellular carcinoma". Proceedings of the National Academy of Sciences of the United States of America 108 (2): 786–791. January 2011. doi:10.1073/pnas.1011098108. PMID 21187392. Bibcode: 2011PNAS..108..786B.
- ↑ "Journal of Clinical Oncology, Special Issue on Molecular Oncology: Receptor-Based Therapy, April 2005". http://www.jco.org/content/vol23/issue11/.
- ↑ "Adjuvant intra-arterial iodine-131-labelled lipiodol for resectable hepatocellular carcinoma: a prospective randomised trial". Lancet 353 (9155): 797–801. March 1999. doi:10.1016/S0140-6736(98)06475-7. PMID 10459961.
- ↑ "Hepatocellular carcinoma: the need for progress". Journal of Clinical Oncology 23 (13): 2892–2899. May 2005. doi:10.1200/JCO.2005.03.196. PMID 15860847.
- ↑ "Human primary liver cancer-derived organoid cultures for disease modeling and drug screening". Nature Medicine 23 (12): 1424–1435. December 2017. doi:10.1038/nm.4438. PMID 29131160.
- ↑ "A novel RNA sequencing-based miRNA signature predicts with recurrence and outcome of hepatocellular carcinoma". Molecular Oncology 12 (7): 1125–1137. June 2018. doi:10.1002/1878-0261.12315. PMID 29719937.
- ↑ Stepien M, Lopez-Nogueroles M, Lahoz A, Kühn T, Perlemuter G, Voican C, Ciocan D, Boutron-Ruault MC, Jansen E, Viallon V, Leitzmann M, Tjønneland A, Severi G, Mancini FR, Dong C, Kaaks R, Fortner RT, Bergmann MM, Boeing H, Trichopoulou A, Karakatsani A, Peppa E, Palli D, Krogh V, Tumino R, Sacerdote C, Panico S, Bueno-de-Mesquita HB, Skeie G, Merino S, Ros RZ, Sánchez MJ, Amiano P, Huerta JM, Barricarte A, Sjöberg K, Ohlsson B, Nyström H, Werner M, Perez-Cornago A, Schmidt JA, Freisling H, Scalbert A, Weiderpass E, Christakoudi S, Gunter MJ, Jenab M. Prediagnostic alterations in circulating bile acid profiles in the development of hepatocellular carcinoma. Int J Cancer. 2022 Apr 15;150(8):1255-1268. doi: 10.1002/ijc.33885. Epub 2022 Jan 11. PMID 34843121
- ↑ Xie G, Wang X, Huang F, Zhao A, Chen W, Yan J, Zhang Y, Lei S, Ge K, Zheng X, Liu J, Su M, Liu P, Jia W. Dysregulated hepatic bile acids collaboratively promote liver carcinogenesis. Int J Cancer. 2016 Oct 15;139(8):1764-75. doi: 10.1002/ijc.30219. Epub 2016 Jun 17. PMID 27273788; PMCID: PMC5493524
- ↑ "ennerex Granted FDA Orphan Drug Designation for Pexa-Vec in Hepatocellular Carcinoma (HCC)". https://www.wsj.com/article/PR-CO-20130508-915494.html.
- ↑ "Enforcement Reports". https://www.accessdata.fda.gov/scripts/opdlisting/oopd/OOPD_Results_2.cfm?Index_Number=457714.
- ↑ "Open-label Phase II clinical trial in 75 patients with advanced hepatocellular carcinoma receiving daily dose of tableted liver cancer vaccine, hepcortespenlisimut-L". Journal of Hepatocellular Carcinoma 4: 59–69. 2017. doi:10.2147/JHC.S122507. PMID 28443252.
- ↑ "Everolimus and pasireotide for advanced and metastatic hepatocellular carcinoma". Investigational New Drugs 33 (2): 505–509. April 2015. doi:10.1007/s10637-015-0209-7. PMID 25613083.
Further reading
- "Clinical features and diagnosis of hepatocellular carcinoma". uptodate. 20 December 2022. http://www.uptodate.com/contents/clinical-features-and-diagnosis-of-primary-hepatocellular-carcinoma?source=see_link.
- "Management of hepatocellular carcinoma". Hepatology 42 (5): 1208–1236. November 2005. doi:10.1002/hep.20933. PMID 16250051.
- "Hepatic Resection for Hepatocellular Carcinoma". The Hong Kong Medical Diary 10 (12): 15–17. December 2005. http://www.fmshk.com.hk/article/596.pdf.
External links
- Blue Faery: The Adrienne Wilson Liver Cancer Association (hepatocellular carcinoma patient support site)
- NCI Liver Cancer Homepage
| Classification | |
|---|---|
| External resources |
 |





