Biology:Naegleria fowleri
This article is missing information about genome assemblies (drafts and one unpublished but finished genome on NCBI). (January 2021) |
| Naegleria fowleri | |
|---|---|
| |
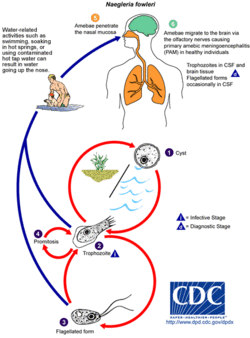
| Diagram depicting the stages of Naegleria fowleri’s life-cycle and environment at that stage | |

| |
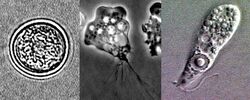
| Drawings of the three stages Naegleria fowleri’s life-cycle | |
| Scientific classification | |
| Domain: | Eukaryota |
| Phylum: | Percolozoa |
| Class: | Heterolobosea |
| Order: | Schizopyrenida |
| Family: | Vahlkampfiidae |
| Genus: | Naegleria |
| Species: | N. fowleri
|
| Binomial name | |
| Naegleria fowleri Carter (1970)
| |
Naegleria fowleri, colloquially known as the "brain-eating amoeba", is a species of the genus Naegleria. It belongs to the phylum Percolozoa and is technically classified as an amoeboflagellate excavate,[1] rather than a true amoeba. This free-living microorganism primarily feeds on bacteria but can become pathogenic in humans, causing an extremely rare, sudden, severe, and usually fatal brain infection known as naegleriasis or primary amoebic meningoencephalitis (PAM).[2]
Typically found in warm freshwater bodies such as ponds or badly managed pools [3] lakes,[4] rivers, hot springs,[5] warm water discharge from industrial or power plants,[6] geothermal well water,[7] poorly maintained or minimally chlorinated swimming pools with residual chlorine levels under 0.5 mg/m3,[8] water heaters,[9] soil, and pipes connected to tap water,[10] it can exist in either an amoeboid or temporary flagellate stage.[11]
Etymology
The organism was named after Malcolm Fowler, an Australian pathologist at Adelaide Children's Hospital, who was the first author of the original series of case reports of PAM.[12][13]
Life cycle
Naegleria fowleri, a thermophilic and free-living amoeba, is primarily found in warm and hot freshwater environments such as ponds, lakes, rivers, and hot springs.[14] As temperatures rise, its population tends to increase. Although the amoeba was initially identified in Australia in the 1960s, it is believed to have evolved in the United States.[15] N. fowleri exists in three forms: cyst, trophozoite (ameboid), and biflagellate. While it does not form cysts in solid human tissue, where only the amoeboid trophozoite stage is present, the flagellate form has been discovered in cerebrospinal fluid.
Cyst stage
To endure harsh environmental conditions, trophozoites transform into microbial cysts,[16] spherical, single-layered structures about 7–15 µm in diameter, enclosing a single cell nucleus.[17] Acting as a resilient capsule, the cyst enables the amoeba to withstand adverse circumstances. Factors triggering cyst formation include food scarcity, overcrowding, desiccation, waste accumulation, and cold temperatures. When conditions improve, the amoeba can emerge through the pore or ostiole at the center of the cyst. N. fowleri has been observed to encyst at temperatures below 10 °C (50 °F).[17][16]
Trophozoite stage
The trophozoite stage is the infective phase for humans, during which the organism can actively feed and replicate. The trophozoite attaches to the olfactory epithelium, follows the axons of olfactory receptor neurons through the cribriform plate in the nasal cavity, and enters the brain. This reproductive stage of the protozoan organism transforms around 25 °C (77 °F), and thrives best at approximately 42 °C (108 °F), multiplying through binary fission.
Trophozoites are characterized by a nucleus surrounded by a flexible membrane. They move via pseudopodia, extending parts of their cell membrane (pseudopods) and filling them with protoplasm to facilitate locomotion. Pseudopods form in the direction of movement. In their free-living state, trophozoites feed on bacteria. In tissues, they appear to phagocytize (enclose and digest) red blood cells and cause tissue damage either through the release of cytolytic substances or by direct cell-to-cell contact using cytolytic membrane proteins.[17]
As trophozoites, Naegleria fowleri may develop approximately 1 to 12 structures on their membrane known as amoebastomes (amorphous cytostomes), also referred to as "suckers" or "food cups," which they use for feeding in a manner similar to trogocytosis.[18]
Flagellate stage
The flagellate stage of Naegleria fowleri is pear-shaped and biflagellate (with two flagella). This stage can be inhaled into the nasal cavity, typically during activities such as swimming or diving. The flagellate form develops when trophozoites are exposed to a change in ionic strength in the fluid it is in, such as being placed in distilled water. The flagellate form does not exist in human tissue, but can be present in the cerebrospinal fluid. Once inside the nasal cavity, the flagellated form transforms into a trophozoite within a few hours.[17]
Ecology
Naegleria fowleri, an excavata, inhabits soil and water. It is sensitive to drying and acidic conditions, and cannot survive in seawater. The amoeba thrives at moderately elevated temperatures, making infections more likely during summer months. N. fowleri is a facultative thermophile, capable of growing at temperatures up to 46 °C (115 °F).[11] Warm freshwater with an ample supply of bacteria as food provides a suitable habitat for amoebae. Locations where many amoebic infections have occurred include artificial bodies of water, disturbed natural habitats, areas with soil, and unchlorinated or unfiltered water.
N. fowleri appears to flourish during periods of disturbance. The "flagellate-empty" hypothesis suggests that Naegleria's success may stem from decreased competition when thermosensitive protozoal fauna do not survive changes in temperature. In other words, N. fowleri thrives when other predators consuming its food supply are absent. This hypothesis implies that human disturbances, such as thermal pollution, increase the abundance of N. fowleri by eliminating its resource competitors. Amoeboflagellates have a motile flagellate stage that aids in dispersal, that is advantageous in environments cleared of competing organisms.
Pathogenicity
N. fowleri may cause a usually fatal infection of the brain called naegleriasis, primary amoebic meningoencephalitis (PAM), amoebic encephalitis/meningitis, or simply Naegleria infection. Infections most often occur when water containing N. fowleri is inhaled through the nose (aspirated), where it then enters the nasal and olfactory nerve tissue, travelling to the brain through the cribriform plate.[19] N. fowleri cannot cause infection by swallowing contaminated water.[20] Infections typically occur after swimming in warm-climate freshwater, although there have been cases in cooler climates such as Minnesota, US.[21] In rare cases, infection has been caused by nasal or sinus rinsing with contaminated water in a nasal rinsing device such as a neti pot.[10]
N. fowleri normally eat bacteria, but during human infections, the trophozoites consume astrocytes and neurons. The reason why N. fowleri passes across the cribriform plate is not known, but the neurotransmitter acetylcholine has been suggested as a stimulus precipitating the action, as a structural homolog of animal CHRM1 has been shown to be present in Naegleria and Acanthamoeba.[22]
It takes one to twelve days, median five, for symptoms to appear after nasal exposure to N. fowleri flagellates.[23] Symptoms may include headache, fever, nausea, vomiting, loss of appetite, altered mental state, coma, drooping eyelid, blurred vision, and loss of the sense of taste.[24] Later symptoms may include stiff neck, confusion, lack of attention, loss of balance, seizures, and hallucinations. Once symptoms begin to appear, the patient usually dies within two weeks. N. fowleri is not contagious; an infected person cannot transmit the infection. From 2013 to 2022, 29 infections were reported in the US, which compares with about 4,000 annual deaths by drowning.[25] It is so rare that individual cases are often reported internationally.[26]
Animals may be infected by Naegleria fowleri. This is rarely observed, although it may occur and be overlooked. Experimentally, mice, guinea pigs, and sheep have been infected, and there have been reports of South American tapirs and cattle contracting PAM.[27]
Treatment
The core antimicrobial treatment consists of the antifungal drug amphotericin B,[28] which inhibits the pathogen by binding to its cell membrane sterols, causing cell membrane disruption and pathogen death;[29] however, even with this treatment, the fatality rate is greater than 97%.[25][30] New treatments are being sought.[25][31] Miltefosine, an antiparasitic drug that inhibits the pathogen via disrupting its cell survival signal pathway PI3K/Akt/mTOR,[29] has been used in a few cases with mixed results.[32]
A key factor to effective treatment is the speed of diagnosis. Naegleriasis is rare, and is often not considered as a likely diagnosis; therefore, the clinical laboratory's identification of the microorganism may be the first time an amoebic etiology is considered. Rapid identification can help to avoid delays in diagnosis and therapy. Amoeba cultures and real-time polymerase chain reaction (PCR) studies for N. fowleri are diagnostic of PAM, but they are not readily available at most institutions, and would have to be carried out at a reference laboratory. The time of presentation of the patient may affect the identification of the microorganism also, as PAM has an incubation period ranging from 1 to 12 days.[25] The clinical signs of PAM are similar to bacterial and viral meningitis, including fever, neck stiffness, and severe headaches. Symptoms can progress to prolonged nausea, vomiting, and even seizures. The disease can progress to acute hemorrhagic necrotizing meningoencephalitis. After symptoms start the patient typically dies within 1 to 18 days, typically about 5 days.[25] A variable delay in treatment can be secondary to time intervals in multiple stages of care, including exposure to exhibition of symptoms; arrival for treatment at a health care facility; workup of the diagnosis (initial diagnosis of likely bacterial meningitis); and finally, from diagnosis to initiation of recommended therapy. Successful treatment of PAM is rare; treatment can only be attempted after correct diagnosis, which relies on rapid recognition of the microorganism by medical technologists and pathologists. It is critical that medical technologists consistently provide timely CSF evaluation, explore the diagnosis of PAM, and look for amoebae in the setting of meningitis, especially in summer.[33]
Cases in Pakistan
In Pakistan the number of reported cases has surpassed the global total due to insufficient healthcare infrastructure and limited awareness of Naegleria fowleri. As a result, only a small fraction of cases are correctly identified as Primary Amebic Meningoencephalitis (PAM), with the majority of cases misdiagnosed as viral meningitis.
For the very first time in Pakistan, N. fowleri genotype has been identified as type-2. Phylogenetic analysis showed that N. fowleri isolate from Pakistan is among the latest descendants, i.e., evolved later in life.[34]
See also
- Acanthamoeba – an amoeba that can cause amoebic keratitis and encephalitis in humans
- Balamuthia mandrillaris – an amoeba that is the cause of (often fatal) granulomatous amoebic meningoencephalitis
- Entamoeba histolytica – an amoeba that is the cause of amoebiasis, or amoebic dysentery
- Leptospira – a zoonotic bacteria that causes leptospirosis
- Methicillin-resistant Staphylococcus aureus (MRSA)
- Necrotizing fasciitis – the "flesh-eating disease", caused by certain types of bacteria
- Toxoplasma gondii – cat-carried protozoan that causes the disease toxoplasmosis
- Vibrio vulnificus – warm saltwater infectious bacteria
References
- ↑ Schuster, Frederick L.; Visvesvara, Govinda S. (2004). "Free-living amoebae as opportunistic and non-opportunistic pathogens of humans and animals". International Journal for Parasitology 34 (9): 1001–1027. doi:10.1016/j.ijpara.2004.06.004. PMID 15313128.
- ↑ "Texas residents warned of tap water tainted with brain-eating microbe". The Guardian. Associated Press. 26 September 2020. https://www.theguardian.com/us-news/2020/sep/26/texas-tap-water-tainted-brain-eating-microbe.
- ↑ Maclean, RebeccaC.; Richardson, DennisJ.; LePardo, Robin; Marciano-Cabral, Francine (2004). "The identification of Naegleria fowleri from water and soil samples by nested PCR". Parasitology Research 93 (3): 211–217. doi:10.1007/s00436-004-1104-x. PMID 15138806.
- ↑ Wellings, F. M.; Amuso, P. T.; Chang, S. L.; Lewis, A. L. (1977). "Isolation and identification of pathogenic Naegleria from Florida lakes.". Appl Environ Microbiol 34 (6): 661–7. doi:10.1128/AEM.34.6.661-667.1977. PMID 596870. Bibcode: 1977ApEnM..34..661W.
- ↑ Sheehan, Kathy B.; Fagg, Jennifer A.; Ferris, Michael J.; Henson, Joan M. (2003). "PCR Detection and Analysis of the Free-Living Amoeba Naegleria in Hot Springs in Yellowstone and Grand Teton National Parks". Applied and Environmental Microbiology 69 (10): 5914–5918. doi:10.1128/AEM.69.10.5914-5918.2003. PMID 14532044. Bibcode: 2003ApEnM..69.5914S.
- ↑ Sykora, J. L.; Keleti, G.; Martinez, A. J. (1983). "Occurrence and pathogenicity of Naegleria fowleri in artificially heated waters.". Appl Environ Microbiol 45 (3): 974–9. doi:10.1128/AEM.45.3.974-979.1983. PMID 6847189. Bibcode: 1983ApEnM..45..974S.
- ↑ Marciano-Cabral, Francine; MacLean, Rebecca; Mensah, Alex; LaPat-Polasko, Laurie (2003). "Identification of Naegleria fowleri in Domestic Water Sources by Nested PCR". Applied and Environmental Microbiology 69 (10): 5864–5869. doi:10.1128/AEM.69.10.5864-5869.2003. PMID 14532037. Bibcode: 2003ApEnM..69.5864M.
- ↑ Yoder, J. S.; Eddy, B. A.; Visvesvara, G. S.; Capewell, L.; Beach, M. J. (2009). "The epidemiology of primary amoebic meningoencephalitis in the USA, 1962–2008". Epidemiology and Infection 138 (7): 968–975. doi:10.1017/S0950268809991014. PMID 19845995.
- ↑ Yoder, J. S.; Straif-Bourgeois, S.; Roy, S. L.; Moore, T. A.; Visvesvara, G. S.; Ratard, R. C.; Hill, V. R.; Wilson, J. D. et al. (2012). "Primary Amebic Meningoencephalitis Deaths Associated With Sinus Irrigation Using Contaminated Tap Water". Clinical Infectious Diseases 55 (9): e79–e85. doi:10.1093/cid/cis626. PMID 22919000.
- ↑ 10.0 10.1 "Naegleria fowleri — Primary Amebic Meningoencephalitis (PAM): Ritual Nasal Rinsing & Ablution". CDC. 2023-05-03. https://www.cdc.gov/parasites/naegleria/ritual-ablution.html.
- ↑ 11.0 11.1 "Naegleria fowleri — Primary Amebic Meningoencephalitis (PAM): General Information". Centers for Disease Control and Prevention (CDC). 2023-05-03. https://www.cdc.gov/parasites/naegleria/general.html.
- ↑ Fowler, M.; Carter, R. F. (September 1965). "Acute pyogenic meningitis probably due to Acanthamoeba sp.: a preliminary report". British Medical Journal 2 (5464): 740–2. doi:10.1136/bmj.2.5464.734-a. PMID 5825411.
- ↑ "The discovery of amoebic meningitis in Northern Spencer Gulf towns". South Australian Medical Heritage Society Inc. https://www.samhs.org.au/Virtual%20Museum/Medicine/amoeb-mening/amoebic-miningitis.htm.
- ↑ "Identification of Naegleria fowleri in warm ground water aquifers". Journal of Environmental Quality 39 (1): 147–153. January 2010. doi:10.2134/jeq2009.0062. PMID 20048302. Bibcode: 2010JEnvQ..39..147L.
- ↑ "Brain-eating-amoeba". WebMD. http://www.webmd.com/brain/brain-eating-amoeba.
- ↑ 16.0 16.1 Chang, S.L. (1978). "Resistance of pathogenic Naegleria to some common physical and chemical agents". Applied and Environmental Microbiology 35 (2): 368–375. doi:10.1128/AEM.35.2.368-375.1978. PMID 637538. Bibcode: 1978ApEnM..35..368C.
- ↑ 17.0 17.1 17.2 17.3 Marciano-Cabral, F (1988). "Biology of Naegleria spp". Microbiological Reviews 52 (1): 114–133. doi:10.1128/MMBR.52.1.114-133.1988. PMID 3280964.
- ↑ John, D T; Cole, T B; Marciano-Cabral, F M (January 1984). "Sucker-like structures on the pathogenic amoeba Naegleria fowleri" (in en). Applied and Environmental Microbiology 47 (1): 12–14. doi:10.1128/aem.47.1.12-14.1984. ISSN 0099-2240. PMID 6696410. Bibcode: 1984ApEnM..47...12J.
- ↑ Baig, AM (Aug 2015). "Pathogenesis of amoebic encephalitis: Are the amoebae being credited to an 'inside job' done by the host immune response?". Acta Trop. 148: 72–76. doi:10.1016/j.actatropica.2015.04.022. PMID 25930186.
- ↑ "Primary Amebic Meningoencephalitis (PAM) - Naegleria fowleri | Parasites | CDC". 2019-06-24. https://www.cdc.gov/parasites/naegleria/index.html.
- ↑ "Naegleria and Amebic Meningoencephalitis – Minnesota Dept. of Health". https://www.health.state.mn.us/diseases/naegleria/index.html.
- ↑ Baig, AM (Aug 2016). "Primary Amoebic Meningoencephalitis: Neurochemotaxis and Neurotropic Preferences of Naegleria fowleri". ACS Chem Neurosci 7 (8): 1026–1029. doi:10.1021/acschemneuro.6b00197. PMID 27447543.
- ↑ "Naegleria fowleri – Primary Amebic Meningoencephalitis (PAM): Illness & Symptoms". Centers for Disease Control and Prevention (CDC). May 3, 2023. https://www.cdc.gov/parasites/naegleria/illness.html.
- ↑ "Brain-Eating Amoeba (Naegleria Fowleri): FAQ, Symptoms, Treatment". 2021-09-29. https://www.webmd.com/brain/brain-eating-amoeba.
- ↑ 25.0 25.1 25.2 25.3 25.4 "Frequently asked questions about Naegleria fowleri, commonly known as the "brain-eating ameba"". Centers for Disease Control and Prevention (CDC). May 3, 2023. https://www.cdc.gov/parasites/naegleria/general.html.
- ↑ Helmore, Edward (19 September 2023). "Arkansas child dies of rare brain-eating amoeba after playing at country club". The Guardian (UK). https://www.theguardian.com/us-news/2023/sep/19/arkansas-child-dies-brain-eating-amoeba-naegleria-fowleri.
- ↑ "Naegleria Fowleri in Animals". Infectious Disease Epidemiology Section, Office of Public Health, Louisiana Dept of Health & Hospitals. 25 September 2013. https://ldh.la.gov/assets/oph/Center-PHCH/Center-CH/infectious-epi/EpiManual/NaegleriaFowleriInAnimalsManual.pdf.
- ↑ Subhash Chandra Parija (Nov 23, 2015). "Naegleria Infection Treatment & Management". Medscape. http://emedicine.medscape.com/article/223910-treatment.
- ↑ 29.0 29.1 Asbill, Scott; Virga, Kris (2015). "Naegleria Fowleri: Pathogenesis, Diagnosis, and Treatment Options". Antimicrobial Agents and Chemotherapy 59 (11): 6677–6681. doi:10.1128/AAC.01293-15. PMID 26259797.
- ↑ Cetin, N; Blackall, D (April 2012). "Naegleria fowleri meningoencephalitis". Blood 119 (16): 3658. doi:10.1182/blood-2011-06-353136. PMID 22645743.
- ↑ Wessel, Lindzi (22 July 2016). "Scientists scour the globe for a drug to kill deadly brain-eating amoeba". STAT. https://www.statnews.com/2016/07/22/brain-eating-amoeba/.
- ↑ Wessel, Linda (16 September 2016). "A life-saving drug that treats a rare infection is almost impossible to find". Business Insider. http://www.businessinsider.com/why-brain-eating-amoeba-miltefosine-medicine-is-hard-to-find-2016-9?amp.
- ↑ Pugh, J. Jeffrey; Levy, Rebecca A. (2016-09-21). "Naegleria fowleri: Diagnosis, Pathophysiology of Brain Inflammation, and Antimicrobial Treatments". ACS Chemical Neuroscience 7 (9): 1178–1179. doi:10.1021/acschemneuro.6b00232. PMID 27525348.
- ↑ Aurongzeb, Muhammad; Rashid, Yasmeen; Ahmed Naqvi, Syed Habib; Khatoon, Ambrina; Abdul Haq, Sadia; Azim, Mohammad Kamran; Kaleem, Imdad; Bashir, Shahid (2022). "Naegleria fowleri from Pakistan Has Type-2 Genotype". Iranian Journal of Parasitology 17 (1): 43–52. doi:10.18502/ijpa.v17i1.9015. ISSN 1735-7020. PMID 36046566.
External links
- Naegleria information site from the Centers for Disease Control and Prevention
- Naegleria from The Tree of Life Web Project
Wikidata ☰ Q131273 entry
 |