Biology:Balamuthia mandrillaris
| Balamuthia mandrillaris | |
|---|---|
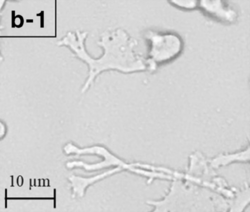
| |
| Trophozoite (active) form of Balamuthia mandrillaris | |

| |
| A Balamuthia mandrillaris cyst | |
| Scientific classification | |
| Domain: | Eukaryota
|
| Phylum: | |
| Class: | |
| Order: | |
| Family: | Balamuthiidae
|
| Genus: | Balamuthia Visvesvara et al., 1993
|
| Species: | B. mandrillaris
|
| Binomial name | |
| Balamuthia mandrillaris Visvesvara et al., 1993
| |
Balamuthia mandrillaris is a free-living amoeba that causes the rare but deadly neurological condition granulomatous amoebic encephalitis (GAE).[1] B. mandrillaris is a soil-dwelling amoeba and was first discovered in 1986 in the brain of a mandrill that died in the San Diego Wild Animal Park.[2][3]
B. mandrillaris can infect the body through open wounds or possibly by inhalation.[4] Balamuthia has been isolated from soil.[5][6] It is believed to be distributed throughout the temperate regions of the world. This is supported somewhat by the detection of antibodies to the protist in healthy individuals.
The generic name Balamuthia was given by Govinda Visvesvara, after his mentor, parasitologist William Balamuth, for his contributions to the study of amoebae. Visvesvara isolated and studied the pathogen for the first time in 1993.[7]
Morphology
B. mandrillaris is a free-living, heterotrophic amoeba, consisting of a standard complement of organelles surrounded by a three-layered cell wall (thought to be made of cellulose), and with an abnormally large cell nucleus. On average, a Balamuthia trophozoite is about 30 to 120 μm in diameter. The cysts fall around this range, as well.[8]
Life cycle
Balamuthia's lifecycle, like the Acanthamoeba, consists of a cystic stage and a non-flagellated trophozoite stage, both of which are infectious, and both of which can be identified in the brain tissue on microscopic examination of brain biopsies performed on infected individuals. The trophozoite is pleomorphic and uninucleated, but binucleated forms are occasionally seen. Cysts are also uninucleated, possessing three walls: an outer thin irregular ectocyst, an inner thick endocyst, and a middle amorphous fibrillar mesocyst.[9]
Pathology
B. mandrillaris is larger than human leukocytes, thus making phagocytosis impossible. Instead, the immune system attempts to contain them at the portal of entry (usually an open wound) by mounting a type IV hypersensitivity reaction.[10] Upon introduction, the amoeba may form a skin lesion, or in some cases, may migrate to the brain, causing a condition known as granulomatous amoebic encephalitis (GAE),[11] which is usually fatal. This granulomatous feature is mostly seen in immunocompetent patients; immunocompromised individuals exhibit a "perivascular cuffing".[12] Balamuthia-induced GAE can cause focal paralysis, seizures, and brainstem symptoms such as facial paralysis, difficulty swallowing, and double vision.[13]
Balamuthia may also cause a variety of non-neurological symptoms, including skin lesions, which can progress to GAE. Patients experiencing this particular syndrome may report a skin lesion (often similar to those caused by MRSA), which does not respond well to antibiotics. The lesion is usually localized and very slow to heal, or fails to heal altogether. In some presentations, this infection may be mistaken for certain forms of skin cancer or cutaneous leishmaniasis. Balamuthia lesions are most often painless.[13]
Culturing and identification
Biopsies of skin lesions, sinuses, lungs, and the brain can detect of B. mandrillaris infection. The amoeba cannot be cultured on an agar plate coated with E. coli because, unlike Naegleria or Acanthamoeba, Balamuthia mandrillaris does not feed on bacteria. Instead, Balamuthia must be cultured on primate hepatocytes or human brain microvascular endothelial cells.[14] Formalin-fixed paraffinized biopsy specimens may indicate Balamuthia trophozoites in the perivascular space. The cysts can be visualized by calcofluor white, which binds to glycans on the cyst wall. Trophozoites appear circular during infection.[13]
Vero cells have been suggested as a possible cheaper and faster alternative to culture the organism.[15] Several types of animal cells have been used in B. mandrillaris culturing including rat glioma cells, human lung cells, and human brain microvascular endothelial cells.[13] These animal cells are added to a specified axenic growth medium for culturing. At the same time, and xenic culture is also performed to help differentiate between Balamuthia and other amebae.[13]
Treatment
Infection seems to be survivable if treated early. Two individuals, a 5-year-old girl and a 64-year-old man, developed GAE. After diagnosis, they were treated with flucytosine, pentamidine, fluconazole, sulfadiazine, a macrolide antibiotic and trifluoperazine. Both patients recovered.[16] In 2018, an unsuccessful attempt at treatment of a Balamuthia infection after nasal lavage with untreated tap water was reported.[17]
Nitroxoline has shown interesting properties in vitro and might be a possible treatment for this infection.[18] A man treated with nitroxoline at UCSF Medical Center in 2021, following a seizure that was identified to have resulted from Balamuthia mandrillaris granulomatous amebic encephalitis, survived and recovered from the disease, indicating that nitroxoline might be a promising medication.[19][20]
Organ transplantation
According to a report published in Morbidity and Mortality Weekly Report in September 2010, two confirmed cases of Balamuthia transmission occurred through organ transplantation in December 2009 in Mississippi.[21] Two kidney recipients, a 31-year-old woman and a 27-year-old man, suffered from post-transplant encephalitis due to Balamuthia. The woman died in February 2010 and the man survived with partial paralysis of his right arm. The CDC was notified by a physician on December 14, 2009, about possible transplant transmission in these two patients. Histopathologic testing of donor and recipient tissues confirmed the transmission. Two other patients who received heart and liver transplants from the same donor, but in different hospitals, were placed on preemptive therapy and remain unaffected. A second cluster of transplant-transmitted Balamuthia in Arizona was reported in the same weekly report. Four recipients were identified, two from Arizona (liver and kidney-pancreas), one from California (kidney), and another from Utah (heart). Recipients from Arizona—a 56-year-old male and a 24-year-old male—both succumbed to GAE within a span of 40 days from transplantation. The other two were placed on preemptive therapy after the first two were reported and remain unaffected.[22]
References
- ↑ Sarica, Feyzi Birol; Tufan, Kadir; Cekinmez, Melih; Erdoğan, Bülent; Altinörs, Mehmet Nur (2009). "A rare but fatal case of granulomatous amebic encephalitis with brain abscess: the first case reported from Turkey". Turkish Neurosurgery 19 (3): 256–259. PMID 19621290. https://pubmed.ncbi.nlm.nih.gov/19621290/.
- ↑ Cope, Jennifer R.; Landa, Janet; Nethercut, Hannah; Collier, Sarah A.; Glaser, Carol; Moser, Melanie; Puttagunta, Raghuveer; Yoder, Jonathan S. et al. (2019-05-17). "The Epidemiology and Clinical Features of Balamuthia mandrillaris Disease in the United States, 1974 – 2016". Clinical Infectious Diseases 68 (11): 1815–1822. doi:10.1093/cid/ciy813. ISSN 1058-4838. PMID 30239654.
- ↑ Visvesvara, G S; Martinez, A J; Schuster, F L; Leitch, G J; Wallace, S V; Sawyer, T K; Anderson, M (1990-12-28). "Leptomyxid ameba, a new agent of amebic meningoencephalitis in humans and animals" (in en). Journal of Clinical Microbiology 28 (12): 2750–2756. doi:10.1128/jcm.28.12.2750-2756.1990. ISSN 0095-1137. PMID 2280005.
- ↑ "Balamuthia mandrillaris ameba infection". Centers for Disease Control and Prevention. https://www.cdc.gov/Features/organsafety/.
- ↑ Frederick L. Schuster; Thelma H. Dunnebacke; Gregory C. Booton; Shigeo Yagi; Candice K. Kohlmeier; Carol Glaser; Duc Vugia; Anna Bakardjiev et al. (July 2003). "Environmental Isolation of Balamuthia mandrillaris Associated with a Case of Amebic Encephalitis". J. Clin. Microbiol. 41 (7): 3175–3180. doi:10.1128/JCM.41.7.3175-3180.2003. PMID 12843060.
- ↑ Thelma H. Dunnebacke; Frederick L. Schuster; Shigeo Yagi; Gregory C. Booton (September 2004). "Balamuthia mandrillaris from soil samples". Microbiology 150 (Pt 9): 2837–2842. doi:10.1099/mic.0.27218-0. PMID 15347743. http://www.microbiologyresearch.org/docserver/fulltext/micro/150/9/2837.pdf?expires=1497917872&id=id&accname=guest&checksum=5EDA3517D86116ABEB86E9F02177EE27. Retrieved 2017-06-20.
- ↑ Kaneshiro, E. S.; Marciano-Cabral, F.; Moura, H. (2014). "Govinda S. Visvesvara: A Tribute". The Journal of Eukaryotic Microbiology 62 (1): 1–2. doi:10.1111/jeu.12143. PMID 25040661.
- ↑ Ruqaiyyah Siddiqui; Naveed Ahmed Khan (2015). "Balamuthia mandrillaris: Morphology, biology and virulence". Trop. Parasitol. 5 (1): 15–22. doi:10.4103/2229-5070.149888. PMID 25709948.
- ↑ Tropical Infectious Diseases:Principles, Pathogens and Practice (3rd ed.). Saunders. 2011. ISBN 978-0-7020-3935-5. https://books.google.com/books?id=A7GVvFh4WZwC&q=Guerrant+balamuthia&pg=PT5615. Retrieved 6 September 2016.
- ↑ Abdul Mannan Baig. Pathogenesis of amoebic encephalitis: Are the amoebas being credited to an 'inside job' done by the host immune response? Acta Trop. 2015 Apr
- ↑ kfggbhnm Di Gregorio, C; Rivasi F; Mongiardo N; De Rienzo B; Wallace S; Visvesvara GS (December 1992). "Acanthamoeba meningoencephalitis in a patient with acquired immunodeficiency syndrome". Archives of Pathology & Laboratory Medicine 116 (12): 1363–5. PMID 1456885. https://pubmed.ncbi.nlm.nih.gov/1456885/.
- ↑ Mannan Baig, Abdul (Dec 2014). "Granulomatous amoebic encephalitis: ghost response of an immunocompromised host?". J Med Microbiol 63 (12): 1763–6. doi:10.1099/jmm.0.081315-0. PMID 25239626.
- ↑ 13.0 13.1 13.2 13.3 13.4 Bhosale, Namrata K.; Parija, Subhash Chandra (2021). "Balamuthia mandrillaris: An opportunistic, free-living ameba – An updated review". Tropical Parasitology 11 (2): 78–88. doi:10.4103/tp.tp_36_21. ISSN 2229-5070. PMID 34765527.
- ↑ "Balamuthia mandrillaris infection". J. Med. Microbiol. 50 (3): 205–7. March 2001. doi:10.1099/0022-1317-50-3-205. PMID 11232763.
- ↑ Greninger, Alexander L.; Messacar, Kevin; Dunnebacke, Thelma; Naccache, Samia N.; Federman, Scot; Bouquet, Jerome; Mirsky, David; Nomura, Yosuke et al. (2015). "Clinical metagenomic identification of Balamuthia mandrillaris encephalitis and assembly of the draft genome: the continuing case for reference genome sequencing". Genome Medicine 7 (1): 113. doi:10.1186/s13073-015-0235-2. ISSN 1756-994X. PMID 26620704.
- ↑ Deetz, T. R.; Sawyer, M. H.; Billman, G.; Schuster, F. L.; Visvesvara, G. S. (15 November 2003). "Successful Treatment of Balamuthia Amoebic Encephalitis: Presentation of 2 Cases". Clinical Infectious Diseases 37 (10): 1304–1312. doi:10.1086/379020. PMID 14583863.
- ↑ Piper, Keenan H.; Foster, Haidn; Susanto, Daniel; Maree, Cynthia L.; Thornton, Sean D.; Cobbs, Charles S. (December 2018). "Fatal Balamuthia mandrillaris brain infection associated with improper nasal lavage". International Journal of Infectious Diseases 77: 18–22. doi:10.1016/j.ijid.2018.09.013. PMID 30243910.
- ↑ Laurie, Matthew T.; White, Corin V.; Retallack, Hanna; Wu, Wesley; Moser, Matthew S.; Sakanari, Judy A.; Ang, Kenny; Wilson, Christopher et al. (2018). "Functional Assessment of 2,177 U.S. and International Drugs Identifies the Quinoline Nitroxoline as a Potent Amoebicidal Agent against the Pathogen Balamuthia mandrillaris". mBio 9 (5). doi:10.1128/mBio.02051-18. ISSN 2150-7511. PMID 30377287.
- ↑ Kornei, Katherine (2023). "Repurposed drug battles 'brain-eating' amoeba". Science. doi:10.1126/science.adh0048. https://doi.org/10.1126/science.adh0048.
- ↑ Spottiswoode, Natasha; Pet, Douglas; Kim, Annie; Gruenberg, Katherine; Shah, Maulik; Ramachandran, Amrutha; Laurie, Matthew T; Zia, Maham et al. (2023). "Successful Treatment of Balamuthia mandrillaris Granulomatous Amebic Encephalitis with Nitroxoline". Emerging Infectious Diseases 29 (1): 197–201. doi:10.3201/eid2901.221531. PMID 36573629.
- ↑ Centers for Disease Control Prevention (CDC) (17 September 2010). "Balamuthia mandrillaris transmitted through organ transplantation --- Mississippi, 2009.". Morbidity and Mortality Weekly Report 59 (36): 1165–70. PMID 20847719. https://www.cdc.gov/mmwr/preview/mmwrhtml/mm5936a1.htm.
- ↑ Centers for Disease Control Prevention (CDC) (17 September 2010). "Notes from the field: transplant-transmitted Balamuthia mandrillaris --- Arizona, 2010.". Morbidity and Mortality Weekly Report 59 (36): 1182. PMID 20847722. https://www.cdc.gov/mmwr/preview/mmwrhtml/mm5936a1.htm.
- Baig, Abdul Mannan. "Can Neurotropic Free-Living Amoeba Serve as a Model to Study SARS-CoV-2 Pathogenesis?" ACS Chemical Neuroscience., vol. 11, no. 22, 2020, pp. 3697–3700., doi:10.1021/acschemneuro.0c00653.
External links
- Balamuthia | Parasites | CDC for images: Cyst of B. mandrillaris and Trophozoite of B. mandrillaris in culture. Credit: DPDx
Wikidata ☰ Q1159908 entry
 |

