Biology:ORF3b
ORF3b is a non-structural protein which is usually located in the nucleolus or in the mitochondrion of the cell. This protein is encoded by ORF3b gene and that gene is found in the DNA of SARS-CoV and SARS-CoV-2.[1] The infection caused by SARS-CoV-2 is COVID-19, and the one produced by SARS-CoV is SARS, so they have a different immune response. The response produced by COVID-19 is charactarized by a poor induction of a 1-Interferon (IFN-1), poorer than the one produced by SARS, and the reason is not clear. These proteins are being studied to try to understand why they are related to the low induction of interferon-1.[1] Also, it can be a really accurate serological marker of early and late SARS-CoV-2 infection.[2]
Structure
The open reading frame is the part of a gene that has the potential to be translated into a protein. An ORF is a continuous stretch of codons containing a start and stop codon. The "3b" refers to the specific (essential) gene that takes part in the production of the protein. These genes can be found codifying the viral structural proteins S, E, M and N, because they are involved in assembling infectious viruses.
We can find ORF3a and ORF3b gene between the S ("spike protein") and E ("envelope protein").[3] region. Both proteins share a large part of their coding sequence, but their last amino acids (135-154) are different. This difference is found in the C-terminal end, leading them to have different functions.[4] The ORF3b's C-terminal end (from amino acid 134 to 154), is the one to have a "nuclear localization signal". These genes encode different proteins despite having similar sequences. In the case of ORF3b, it can be said that this gene is an "IFN antagonist" because of its ability to mediate in the IRF3 regulatory system. [5]
The protein "3b", when first was studied, was located in the mitochondrial compartment and in the nucleus -nucleolus-. Nonetheless, after using a GFP-tagged[6] expression, it was shown that indeed it could be found in the nucleus -as previously explained- but there was no trace of the presence of this protein in the mitochondria.
Recently, it has been shown that the length of ORF3b determines its ability to suppress an interferon response.[7]
Peptide properties (1-60) [8]:
•Sequence: MMPTTLFAGTHITMTTVYHITVSQIQLSLLKVTAFQHQNSKKTTKLVVILRIGTQVLKTM
•Length: 60
•Mass: 6739.7067
•Isoelectric point (pI): 11.17
•Net charge: +6
•Hydrophobicity: +19.30 Kcal * mol -1
•Extinction coefficient1: 1490 M-1 * cm-1
•Extinction coefficient2: 1490 M-1 * cm-1
Peptide properties (60-120):
•Sequence: SLYMAISPKFTTSLSLHKLLQTLVLKMLHSSSLTSLLKTHRMCKYTQSTALQELLIQQWI
•Length: 60
•Mass: 6901.7382
•Isoelectric point (pI): 10.34
•Net charge: +5
•Hydrophobicity: +16.64 Kcal * mol -1
•Extinction coefficient1: 8480 M-1 * cm-1
•Extinction coefficient2: 8480 M-1 * cm-1
Peptide properties (120-154)
•Sequence: QFMMSRRRLLACLCKHKKVSTNLCTHSFRKKQVR
•Length: 34
•Mass: 4133.2093
•Isoelectric point (pI): 11.97
•Net charge: +10
•Hydrophobicity: +29.64 Kcal * mol -1
•Extinction coefficient1: 125 M-1 * cm-1
•Extinction coefficient2: 0 M-1 * cm-1
Biological processes
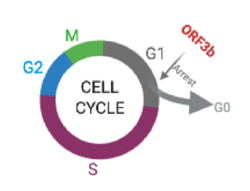
Apoptosis and G0/G1 arrest
Experiments with 3b-EGFP transferred to both monkey and human cells showed that 3b protein induces G0/G1 arrest and apoptosis.[9]
ORF 3b protein was marked with GFP. Flow cytometry performed in those cells (see results in the above histogram, with 3b-EGFP cells labeled as "positive") showed a highly variant distribution of the cell cycle phase between transferred and non-transferred cells, with the first ones presenting an increase in G0/G1 phase as well as a decrease of S phase, which indicates G0/G1 arrest (stopping point in the cell cycle, as it no longer duplicates). In addition, positive cells had a higher percentage of SubG1 phase, an indicator for apoptotic cells.
Another factor that reaffirms this fact is that transferred cells had a larger concetration of Annexin V, which is a protein used to detect apoptotic cells.
Interferon antagonist
With the ongoing COVID-19 pandemic, some studies have shown that the presence of SARS-CoV-2 can lead to impaired interferon (IFN) responses.[1] Its regulatory factor, IRF3, is essential for activating its gene promoter,[10] and most viruses have proteins to inhibit this activation.[11]
Coronaviruses’ proteins 3b also inhibit this process. IRF3b is normally at rest in the cytoplasm, until it is activated and translocates to the nucleus to promote transcriptional activation. What 3b does is inhibating this translocation, therefore not allowing the IFN gene expression.[4]
As previously mentioned, SARS-CoV-2 ORF3b is a much more potent IFN antagonist than other coronaviruses, such as SARS-CoV.
Effect of 3b on the transcriptional activity of AP-1
The AP-1 transcription factor intervenes in many biological processes, such as proliferation, differentiation and cell death.[12] AP-1 also regulates the transcription of many cytokines. Its activity is mainly balanced by the kinases ERK[13] and JNK.[14]
3b protein induces an increase of AP-1 dependent gene expression in 3b-transferred cells.[15] Studies showed that the presence of ORF3b results in an activation of both ERK and JNK signaling, followed by a boost of AP-1 activity. This can lead to a stimulation of some genes related to AP-1, like pro-inflammatory cytokines, which are responsible for the characteristic COVID-19's cytokine storm[16]
Related pathologies
As previously mentioned, ORF3b inhibits the interferon-type-1 molecule that plays a key role in the immune response. This inhibition allows the SARS-CoV-2 to infect many organs quickly, such as lung, liver and immune organ.[2] What's more, this potent interferon antagonist also induces parenchyma cell apoptosis and necrosis.[9]
However, in spite of its pathogenicity, ORF3b can be useful, as it is an accurate serological marker of SARS-CoV-2 infection. Recently, some scientists found out that the combinational use of a test detector of ORF3b and ORF8, another nonstructural protein, could be sufficient, because of its high percentages of sensitivity and specificity, to detect individuals exposed to COVID-19.[17] Furthermore, ORF3b can be detected in a serological test during the incubation period (0-14 days after the infection), which allows infection to be found very early and, consequently, prevent its spread.
SARS-CoV-2 has appeared recently, so much is unknown about this virus and the ORF3b protein to us yet. Even so, now we know that the loss of ORF3b stop codons may be related to the appearance of viral variants with more interferon antagonistic activity.[2]
Also, we know that the most common hosts of this virus are humans, bats and the masked palm civet.[18]
References
- ↑ Jump up to: 1.0 1.1 1.2 Hadjadj, JExpression error: Unrecognized word "et". (7 August 2020). "Impaired type I interferon activity and inflammatory responses in severe COVID-19 patients". Science 369 (6504): 718–724. doi:10.1126/science.abc6027. PMID 32661059.
- ↑ Jump up to: 2.0 2.1 2.2 Konno, Yoriyuki; Kimura, Izumi; Uriu, Keiya; Fukushi, Masaya; Irie, Takashi; Koyanagi, Yoshio; Sauter, Daniel; Gifford, Robert J. et al. (September 2020). "SARS-CoV-2 ORF3b Is a Potent Interferon Antagonist Whose Activity Is Increased by a Naturally Occurring Elongation Variant". Cell Reports 32 (12): 108185. doi:10.1016/j.celrep.2020.108185. ISSN 2211-1247. PMID 32941788.
- ↑ Fernández-Rúa, José M. (13 April 2020). "Nuevo mapa genético del SARS-CoV-2" (in es). https://biotechmagazineandnews.com/nuevo-mapa-genetico-del-sars-cov-2/.
- ↑ Jump up to: 4.0 4.1 Zhou, P; Li, H; Wang, H; Wang, LF; Shi, Z (February 2012). "Bat severe acute respiratory syndrome-like coronavirus ORF3b homologues display different interferon antagonist activities". J Gen Virol 93 (2): 275–281. doi:10.1099/vir.0.033589-0. PMID 22012463.
- ↑ Zhou, Peng; Li, Hongxia; Wang, Hanzhong; Shi, Zhengli (1 February 2012). "Bat severe acute respiratory syndrome-like coronavirus ORF3b homologues display different interferon antagonist activities". Journal of General Virology. https://www.microbiologyresearch.org/docserver/fulltext/jgv/93/2/275_vir033589.pdf?expires=1604916969&id=id&accname=guest&checksum=72D6DE59C6C6AC87624DE24D7063C184.
- ↑ King, Timothy; May, Paul (January 2010). Green Fluorescent Protein - Molecule of the Month January 2010 - HTML-only version. http://www.chm.bris.ac.uk/motm/GFP/GFPh.htm. Retrieved 2 November 2020.
- ↑ Konno, Yoriyuki; Kimura, Izumi; Uriu, Keiya; Fukushi, Masaya; Irie, Takashi; Koyanagi, Yoshio; Nakagawa, So; Sato, Kei (2020-05-12). "SARS-CoV-2 ORF3b is a potent interferon antagonist whose activity is further increased by a naturally occurring elongation variant". http://dx.doi.org/10.1101/2020.05.11.088179.
- ↑ Tulane University (2011). "PepDraw". http://www.tulane.edu/~biochem/WW/PepDraw/.
- ↑ Jump up to: 9.0 9.1 Yuan, X.; Shan, Y.; Chen, J.; Cong, Y. (17 August 2005). "G0/G1 arrest and apoptosis induced by SARS-CoV 3b protein in transfected cells.". Virol J 2 (66): 66. doi:10.1186/1743-422X-2-66. PMID 16107218.
- ↑ Hiscott, J; Pitha, P; Genin, P; Nguyen, H; Heylbroeck, C; Mamane, Y; Algarte, M; Lin, R (19 January 1999). "Triggering the interferon response: the role of IRF-3 transcription factor". J Interferon Cytokine Res 19 (1): 1–13. doi:10.1089/107999099314360. PMID 10048763.
- ↑ Basler, CF; Mikulasova, A; Martinez-Sobrido, L; Paragas, J; Mühlberger, E; Bray, M; Klenk, HD; Palese, P et al. (July 2003). "The Ebola virus VP35 protein inhibits activation of interferon regulatory factor 3.". J Virol 77 (14): 7945–7956. doi:10.1128/jvi.77.14.7945-7956.2003. PMID 12829834.
- ↑ Garces de Los Fayos Alonso, I; Liang, HC; Turner, SD; Lagger, S; Merkel, O; Kenner, L (28 March 2018). "The Role of Activator Protein-1 (AP-1) Family Members in CD30-Positive Lymphomas". Cancers (Basel) 10 (4): 93. doi:10.3390/cancers10040093. PMID 29597249.
- ↑ Frost, JA; Geppert, TD; Cobb, MH; Feramisco, JR (26 April 1994). "A requirement for extracellular signal-regulated kinase (ERK) function in the activation of AP-1 by Ha-Ras, phorbol 12-myristate 13-acetate, and serum". Proc Natl Acad Sci U S A 91 (9): 3844–3848. doi:10.1073/pnas.91.9.3844. PMID 8170999. Bibcode: 1994PNAS...91.3844F.
- ↑ Dérijard, B; Hibi, M; Wu, IH; Barrett, T; Su, B; Deng, T; Karin, M; Davis, RJ (25 March 1994). "JNK1: a protein kinase stimulated by UV light and Ha-Ras that binds and phosphorylates the c-Jun activation domain". Cell 76 (6): 1025–37. doi:10.1016/0092-8674(94)90380-8. PMID 8137421.
- ↑ Varshney, B; Lal, SK (21 June 2011). "SARS-CoV accessory protein 3b induces AP-1 transcriptional activity through activation of JNK and ERK pathways". Biochemistry 50 (24): 5419–5425. doi:10.1021/bi200303r. PMID 21561061.
- ↑ Mehta, P; McAuley, DF; Brown, M; Sanchez, E; Tattersall, RS; Manson, JJ; HLH Across Speciality Collaboration, UK (28 March 2020). "COVID-19: consider cytokine storm syndromes and immunosuppression". Lancet 395 (10229): 1033–1034. doi:10.1016/S0140-6736(20)30628-0. PMID 32192578.
- ↑ Hachim, Asmaa; Kavian, Niloufar; Cohen, Carolyn A.; Chin, Alex W. H.; Chu, Daniel K. W.; Mok, Chris K. P.; Tsang, Owen T. Y.; Yeung, Yiu Cheong et al. (October 2020). "ORF8 and ORF3b antibodies are accurate serological markers of early and late SARS-CoV-2 infection" (in en). Nature Immunology 21 (10): 1293–1301. doi:10.1038/s41590-020-0773-7. ISSN 1529-2916. PMID 32807944. https://www.nature.com/articles/s41590-020-0773-7.
- ↑ "3b - ORF3b protein - Severe acute respiratory syndrome coronavirus (SARS-CoV) - 3b gene & protein" (in en). https://www.uniprot.org/uniprot/P59633.
This article needs additional or more specific categories. (March 2021) |