Biology:PAEP
 Generic protein structure example |
Human placental protein-14 (PP-14), also known as glycodelin, progestogen-associated endometrial protein (PAEP) or pregnancy-associated endometrial alpha-2 globulin is a glycoprotein that inhibits cell immune function and plays an essential role in the pregnancy process. In humans is encoded by the PAEP gene.[1]
Human endometrium synthesizes several proteins under the influence of progesterone. Of these proteins, placental protein 14 (PP-14) is of particular interest. It is synthesized by the glandular cells of endometrium in the luteal phase of menstrual cycle.[2]
The temporal and spatial expression of PP-14 in the female reproductive tract combined with its biological activities suggest that this glycoprotein probably plays an essential physiological role in the regulation of fertilization, implantation and maintenance of pregnancy.[3][4]
Structure
Placental Protein 14 is codified by 180 amino acid but it is thought that 18 of these are supposed signals peptides. The molecular weight of the PP-14 is 20,555, while its mature form is estimated to weigh 18,787. It is encoded by a 1-kilobase-pair mRNA that is expressed in human secretory endometrium and decidua but not in postmenopausal endometrium, placenta, liver, kidney, and adrenals.
The four cysteinyl residues (positions 66, 106, 119, and 160) responsible for intramolecular disulfide bridges in lactoglobulins are all conserved in PP-14. Southern blot analysis of human DNA suggested that PP-14 gene sequences compass some 20 kilobase pairs of the human genomic DNA.[5]
N-terminal amino acid sequence
The N-terminal amino acid sequence of glycodelin is M D I P Q T K Q D L E L P K L A G T W H S M. This sequence can be compared with horse, sheep, goat, bovine and buffalo beta-lactoglobulin. For example, there are 13 identities out of 22 possible matches with horse beta-lactoglobulin.
PAEP gene
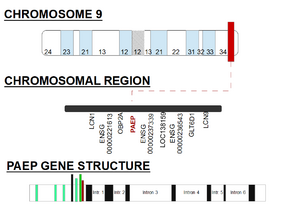
This gene is a member of the kernel lipocalin superfamily whose members share relatively low sequence similarity but have highly conserved exon-intron structure and three-dimensional protein folding. The PAEP gene is clustered on the long arm of chromosome 9 and encodes for PP-14 protein. It is mainly expressed in 60 organs, but reaches its highest expression level in decidua.[6][7]
Function
PP-14 is the most important protein secreted in the endometrium during the mid-luteal phase of the menstrual cycle and during the first semester of pregnancy. Four distinct forms of glycoprotein, with identical protein backbones but different glycosylation profiles, are found in amniotic fluid, follicular fluid and seminal plasma of the reproductive system. These glycoproteins have distinct and essential roles in regulating a uterine environment suitable for pregnancy and in the timing and occurrence of the appropriate sequence of events in the fertilization process.
Glycodelin-A
In the female genital tract is mainly expressed in EECs (cultured endometrial epithelial cells) and secreted into the amniotic fluid, endometrium/decidua and maternal serum. Glycodelin-A has contraceptive and immunosuppressive functions, due to the fact that suppresses Natural Killer cells, achieving the prevention of the maternal rejection of the fetus at the fetromaternal interface. It has a molecular weight of 18.78 KDa determined from the cDNA sequence.[8][9][10]
Glycodelin-S
Is secreted from seminal vesicles to the seminal fluid. A number of alternatively spliced transcript variants have been observed at this locus, but the full-length nature of only two, each encoding the same protein, has been determined. During the passage of the sperm through the cervix, glycodelin S is de-glycosylated and dissociates from the sperm, allowing the sperm to mature.[11][12][13]
Glycodelin-F
Is secreted by granulosa cells into the follicular fluid. Glycodelin-F reduces the blinding of spermatozoa to the zona pellucida which is mainly expressed in the ovary, and synthesised in the granulosa cells, has a function in principle similar to that of Glycodelin-A. It also binds the sperm head, thereby inhibiting acrosome reaction and sperm-egg binding. Upon de-glycosilation, glycodelin F dissociates from the sperm and sperm-egg binding is possible. The de-glycosilation takes place during the passage of the sperm through the corona cell layer. Glycodelin F is thereby important to prevent a premature acrosome reaction.[14][15]
Glycodelin-C
Found in Cumulus Oophorus, stimulates binding of the spermatozoa to the zona pellucida. First, cumulus cells reduce the spermatozoa-zona binding inhibitory activity of follicular fluid probably by taking up and converting glycodelin-A and glycodelin-F into glycodelin-C. Second, spermatozoa have enhanced zona bindig ability after penetrating through the cumulus oophorus. Glycodelin-C is responsible for the latter observation.[16][17]
| ISOFROM | SOURCE | GLYCOSYLATION | REPRODUCTIVE FUNCTIONS |
|---|---|---|---|
| GdA | Amniotic fluid, pregnancy decidua | High sialylation, more fucosylation | Immunoprotection for implantation and placentation, antifertilizing, inhibiting spermatozoa-zona pellucida binding |
| GdS | Seminal plasma, seminal vesicles | No sialylated glycans, rich in fucose and mannose | Preventing premature capacitation |
| GdF | Ovarian follicles, oviduct | Fucosylated Lewis-x and Lewis-y, more N-acetylglucosamine | Inhibiting spermatozoa-zona pellucida, preventing premature acrosome reaction |
| GdC | Cumulus oophorus, converted from GdA and GdF | Reacting with specific agglutinins in lectin-binding manner | Stimulating spermatozoa-zona pellucida binding |
Level concentrations
PP-14 is found in the oocyte and in the sperm. In men, the concentration of this protein in seminal plasma is higher than those in serum. In women, the levels in follicular fluid exceed those of non-pregnant women.[19]
·Seminal plasma:
PP-14 is a significant protein constituent in most seminal plasma samples of men; sometimes comprising over 2.5% of the total protein content. The concentration of PP-14 in seminal plasma from men with oligospermia is in the reference range of this protein derived from values measured in normal men. However, vasectomized men concentrations are less than normal.[20]
·Women's tissues and body fluids:
In serum of non-pregnant women, the concentration of PP-14 is approximately 15-40 µg/L.
In normal pregnancy:
| Location (tissues and body fluids) | PP-14 concentrations (approximately) | Time |
| Serum | Up to 2200 µg/L (highest) | 6–12 weeks |
| --- | Decreasing concentrations | After 16 weeks |
| --- | 200 µg/L approx. | 24 weeks (plateaued) |
| Amniotic fluid | 232 mg/L (highest) (higher than those in maternal serum throughout pregnancy) | 12–20 weeks |
| Cord blood | 15-22 µg or undetectable | ------------ |
| Early pregnancy decidua | 41–160 mg/g total protein | ------------ |
| Late pregnancy decidua | 60-2700 µg/g total protein | ------------ |
| Amnion and chorion laeve | From 50 to 750 µg/g protein | ------------ |
| From 50 to 1000 µg/g protein | ------------ | |
| Early pregnancy placenta | 0.25–15 mg/g | ------------ |
| Late pregnancy placenta | 3-430 µg/g protein | ------------ |
The concentrations of PP-14 in pregnancy serum are comparable with hCG (Human Chorionic Gonadotropin). Among all the placental proteins, the amniotic fluid PP-14 concentration is the most outstanding as decidua is a source of this protein.[21]
Future clinical applications
Placental Protein 14 has some clinical applications:
1. Biomarker of premature rupture of membranes
Premature rupture of membranes is a common pregnancy complication, taking into account that the current method does not satisfy the medical community, some researches have determined a new method: the analysis of placental protein in the maternal plasma and vaginal fluid. The results of these studies have shown that PP-14's concentration increased in the case of premature rupture of membranes. So this study conclude that PP-14 is an excellent biomarker with a sensibility of 100% and a specificity of 87,5%.[22]
2. Biomarker in in vitro fertilization process
PP-14 is known to be a great marker to predict the outcome of in vitro fertilization and the embryo transfer cycle. Some studies have shown that the serum concentration of Placental Protein 14 was highly increased after the embryo transfer cycle, and they conclude that PP-14 might be an excellent marker to predict the endometrial receptivity.[23]
References
- ↑ Wang, Ping; Libho, Zhu; Xinmei, Zhang (December 2013). "The role of Placental Protein 14 in the Pathogenesis of Endometrosis". Reproductive Sciences 20 (12): 1465–1470. doi:10.1177/1933719113488452. ISSN 0077-8923. PMID 23670949.
- ↑ SEPPÄLÄ, MARKKU; JULKUNEN, MERVI; KOSKIMIES, AARNE; LAATIKAINEN, TIMO; STENMAN, ULF–HÅKAN; HUHTALA, MARJA-LIISA (October 1988). "Proteins of the Human Endometrium .". Annals of the New York Academy of Sciences 541 (1 In Vitro Fert): 432–444. doi:10.1111/j.1749-6632.1988.tb22280.x. ISSN 0077-8923. PMID 3195927. Bibcode: 1988NYASA.541..432S.
- ↑ Dutta, Binita; Mukhopadhyay, Debaditya; Roy, Nita; Das, Goutam; Karande, Anjali A. (December 1998). "Cloning, Expression, Purification, and Immunocharacterization of Placental Protein-14". Protein Expression and Purification 14 (3): 327–334. doi:10.1006/prep.1998.0961. ISSN 1046-5928. PMID 9882566.
- ↑ Julkunen, M.; Seppala, M.; Janne, O. A. (1988-12-01). "Complete amino acid sequence of human placental protein 14: a progesterone-regulated uterine protein homologous to beta-lactoglobulins.". Proceedings of the National Academy of Sciences 85 (23): 8845–8849. doi:10.1073/pnas.85.23.8845. ISSN 0027-8424. PMID 3194393. Bibcode: 1988PNAS...85.8845J.
- ↑ Julkunen, M.; Seppala, M.; Janne, O. A. (1988-12-01). "Complete amino acid sequence of human placental protein 14: a progesterone-regulated uterine protein homologous to beta-lactoglobulins.". Proceedings of the National Academy of Sciences 85 (23): 8845–8849. doi:10.1073/pnas.85.23.8845. ISSN 0027-8424. PMID 3194393. Bibcode: 1988PNAS...85.8845J.
- ↑ "PAEP progestagen associated endometrial protein [Homo sapiens (human) - Gene - NCBI"]. https://www.ncbi.nlm.nih.gov/gene?cmd=Retrieve&dopt=full_report&list_uids=5047.
- ↑ "PAEP - Glycodelin precursor - Homo sapiens (Human) - PAEP gene & protein". https://www.uniprot.org/uniprot/P09466.
- ↑ JULKUNEN, MERVI; KOISTINEN, RIITTA; SJÖBERG, JARI; RUTANEN, EEVA-MARJA; WAHLSTRÖM, TORSTEN; SEPPÄLÄ, MARKKU (May 1986). "Secretory Endometrium Synthesizes Placental Protein 14*". Endocrinology 118 (5): 1782–1786. doi:10.1210/endo-118-5-1782. ISSN 0013-7227. PMID 3516653.
- ↑ Kao, L. C.; Tulac, S.; Lobo, S.; Imani, B.; Yang, J. P.; Germeyer, A.; Osteen, K.; Taylor, R. N. et al. (June 2002). "Global Gene Profiling in Human Endometrium during the Window of Implantation". Endocrinology 143 (6): 2119–2138. doi:10.1210/endo.143.6.8885. ISSN 0013-7227. PMID 12021176.
- ↑ Alok, Anshula; Mukhopadhyay, Debaditya; Karande, Anjali A. (May 2009). "Glycodelin A, an immunomodulatory protein in the endometrium, inhibits proliferation and induces apoptosis in monocytic cells". The International Journal of Biochemistry & Cell Biology 41 (5): 1138–1147. doi:10.1016/j.biocel.2008.10.009. ISSN 1357-2725. PMID 18996219.
- ↑ Mandelin, Erik; Koistinen, Hannu; Koistinen, Riitta; Arola, Johanna; Affandi, Biran; Seppälä, Markku (September 2001). "Endometrial expression of glycodelin in women with levonorgestrel-releasing subdermal implants". Fertility and Sterility 76 (3): 474–478. doi:10.1016/s0015-0282(01)01969-0. ISSN 0015-0282. PMID 11532467.
- ↑ Sjöberg, J.; Wahlström, T.; Seppälä, M.; Rutanen, E.-M.; Koistinen, R.; Koskimies, A. I.; Sinosich, M. J.; Teisner, B. et al. (January 1985). "Seminal Plasma Levels of PAPP-A in Normospermic and Oligospermic Men and Tissue Localization of PAPP-A in the Male Genital Tract". Archives of Andrology 14 (2–3): 253–261. doi:10.3109/01485018508988308. ISSN 0148-5016. PMID 2415076.
- ↑ Koistinen, Hannu; Koistinen, Riitta; Dell, Anne; Morris, Howard R.; Easton, Richard L.; Patankar, Manish S.; Oehninger, Sergio; Clark, Gary F. et al. (1996). "Glycodelin from seminal plasma is a differentially glycosylated form of contraceptive glycodelin-A". Molecular Human Reproduction 2 (10): 759–765. doi:10.1093/molehr/2.10.759. ISSN 1360-9947. PMID 9239694.
- ↑ Chiu, Philip C. N.; Chung, Man-Kin; Koistinen, Riitta; Koistinen, Hannu; Seppala, Markku; Ho, Pak-Chung; Ng, Ernest H. Y.; Lee, Kai-Fai et al. (2006-12-27). "Cumulus Oophorus-associated Glycodelin-C Displaces Sperm-bound Glycodelin-A and -F and Stimulates Spermatozoa-Zona Pellucida Binding". Journal of Biological Chemistry 282 (8): 5378–5388. doi:10.1074/jbc.m607482200. ISSN 0021-9258. PMID 17192260.
- ↑ Kölbl, Alexandra C.; Andergassen, Ulrich; Jeschke, Udo (2015-10-13). "The Role of Glycosylation in Breast Cancer Metastasis and Cancer Control". Frontiers in Oncology 5: 219. doi:10.3389/fonc.2015.00219. ISSN 2234-943X. PMID 26528431.
- ↑ Chiu, Philip C. N.; Chung, Man-Kin; Koistinen, Riitta; Koistinen, Hannu; Seppala, Markku; Ho, Pak-Chung; Ng, Ernest H. Y.; Lee, Kai-Fai et al. (2006-12-27). "Cumulus Oophorus-associated Glycodelin-C Displaces Sperm-bound Glycodelin-A and -F and Stimulates Spermatozoa-Zona Pellucida Binding". Journal of Biological Chemistry 282 (8): 5378–5388. doi:10.1074/jbc.m607482200. ISSN 0021-9258. PMID 17192260.
- ↑ Yeung, William S.B.; Lee, Kai-Fai; Koistinen, Riitta; Koistinen, Hannu; Seppälä, Markku; Chiu, Philip C.N. (December 2009). "Effects of glycodelins on functional competence of spermatozoa". Journal of Reproductive Immunology 83 (1–2): 26–30. doi:10.1016/j.jri.2009.04.012. ISSN 0165-0378. PMID 19857900.
- ↑ Cui, Juan; Liu, Yanguo; Wang, Xiuwen (2017-11-29). "The Roles of Glycodelin in Cancer Development and Progression". Frontiers in Immunology 8: 1685. doi:10.3389/fimmu.2017.01685. ISSN 1664-3224. PMID 29238349.
- ↑ SEPPäLä, MARKKU; KOSKIMIES, AARNE I.; TENHUNEN, ANSSI; RUTANEN, EEVA-MARJA; SJÖBERG, JARI; KOISTINEN, RIITTA; JULKUNEN, MERVI; WAHLSTRÖM, TORSTEN (May 1985). "Pregnancy Proteins in Seminal Plasma, Seminal Vesicles, Preovulatory Follicular Fluid, and Ovary". Annals of the New York Academy of Sciences 442 (1 In Vitro Fert): 212–226. doi:10.1111/j.1749-6632.1985.tb37522.x. ISSN 0077-8923. PMID 3893267. Bibcode: 1985NYASA.442..212S.
- ↑ Bolton, A. E.; Pinto-Furtado, L. G.; Andrew, C. E.; Chapman, M. G. (June 1986). "Measurement of the pregnancy-associated proteins, placental protein 14 and pregnancy-associated plasma protein A in human seminal plasma". Clinical Reproduction and Fertility 4 (3): 233–240. ISSN 0725-556X. PMID 2427179.
- ↑ JULKUNEN, MERVI; RUTANEN, EEVA-MARJA; KOSKIMIES, AARNE; RANTA, TAPIO; BOHN, HANS; SEPPALA, MARKKU (November 1985). "Distribution of placental protein 14 in tissues and body fluids during pregnancy". BJOG: An International Journal of Obstetrics and Gynaecology 92 (11): 1145–1151. doi:10.1111/j.1471-0528.1985.tb03027.x. ISSN 1470-0328. PMID 4063232.
- ↑ Yanyun , Haibo, Guanglu, Yanqin, Jun, Qiongli,Qiongli, Linbo ,Tao, Wang, Luo, Che, Li, Gao,Yang, Zhou, Gao, Wang. (2018). "Placental protein 14 as a potential biomarker for diagnosis of preterm premature rupture of membranes". Molecular Medicine Reports 18 (1): 113–122. doi:10.3892/mmr.2018.8967. PMID 29749501. PMC 6059659. https://www.spandidos-publications.com/mmr/18/1/113. Retrieved 25 October 2019.
- ↑ Suzuki, Fukumine, Sugiyama, Usuda, Yoshichika, Noritaka, Rikikazu, Saburo. (2000). "Clinical Applications of Serum Placental Protein 14 (PP14) Measurement in the IVF-ET Cycle". Journal of Obstetrics and Gynaecology Research 26 (4): 295–302. doi:10.1111/j.1447-0756.2000.tb01325.x. PMID 11049241. https://www.researchgate.net/publication/12277696. Retrieved 25 October 2019.
Further reading
- "Glycodelins". Tumour Biology 19 (3): 213–20. 1998. doi:10.1159/000030009. PMID 9591048.
- "Chromosomal location, exon/intron organization and evolution of lipocalin genes". Biochimica et Biophysica Acta (BBA) - Protein Structure and Molecular Enzymology 1482 (1–2): 25–34. Oct 2000. doi:10.1016/S0167-4838(00)00144-8. PMID 11058744.
- "Glycodelin: a reproduction-related lipocalin". Biochimica et Biophysica Acta (BBA) - Protein Structure and Molecular Enzymology 1482 (1–2): 149–56. Oct 2000. doi:10.1016/S0167-4838(00)00158-8. PMID 11058757.
- "Glycodelin: a major lipocalin protein of the reproductive axis with diverse actions in cell recognition and differentiation". Endocrine Reviews 23 (4): 401–30. Aug 2002. doi:10.1210/er.2001-0026. PMID 12202458.
- "Glycosylation related actions of glycodelin: gamete, cumulus cell, immune cell and clinical associations". Human Reproduction Update 13 (3): 275–87. 2007. doi:10.1093/humupd/dmm004. PMID 17329396.
- "Multiple forms of mRNA encoding human pregnancy-associated endometrial alpha 2-globulin, a beta-lactoglobulin homologue". Proceedings of the National Academy of Sciences of the United States of America 88 (6): 2456–60. Mar 1991. doi:10.1073/pnas.88.6.2456. PMID 2006183. Bibcode: 1991PNAS...88.2456G.
- "Serum levels of pregnancy-associated endometrial alpha 2-globulin (alpha 2-PEG), a glycosylated beta-lactoglobulin homologue, in successful and unsuccessful assisted conception". Human Reproduction 5 (4): 421–6. May 1990. doi:10.1093/oxfordjournals.humrep.a137115. PMID 2113930.
- "Human placental protein 14 gene: sequence and characterization of a short duplication". DNA and Cell Biology 9 (6): 401–13. 1990. doi:10.1089/dna.1990.9.401. PMID 2206398.
- "Serum progestagen-associated endometrial protein (PEP) levels in conception versus nonconception cycles following in vitro fertilization-embryo transfer". Journal of in Vitro Fertilization and Embryo Transfer 7 (3): 134–6. Jun 1990. doi:10.1007/BF01135675. PMID 2380618.
- "Immunohistological localization of pregnancy-associated endometrial alpha 2-globulin (alpha 2-PEG) in endometrial adenocarcinoma and effect of medroxyprogesterone acetate". British Journal of Obstetrics and Gynaecology 95 (12): 1292–8. Dec 1988. doi:10.1111/j.1471-0528.1988.tb06820.x. PMID 2975952.
- "Progestin-dependent human endometrial protein: a marker for monitoring human endometrial function". Cell and Molecular Biology of the Uterus. 230. 1988. 167–86. doi:10.1007/978-1-4684-1297-0_10. ISBN 978-1-4684-1299-4.
- "Complete amino acid sequence of human placental protein 14: a progesterone-regulated uterine protein homologous to beta-lactoglobulins". Proceedings of the National Academy of Sciences of the United States of America 85 (23): 8845–9. Dec 1988. doi:10.1073/pnas.85.23.8845. PMID 3194393. Bibcode: 1988PNAS...85.8845J.
- "Amino acid sequence homology between human placental protein 14 and beta-lactoglobulins from various species". Endocrinology 120 (6): 2620–2. Jun 1987. doi:10.1210/endo-120-6-2620. PMID 3569148.
- "Pregnancy-associated endometrial alpha 2-globulin, the major secretory protein of the luteal phase and first trimester pregnancy endometrium, is not glycosylated prolactin but related to beta-lactoglobulins". The Journal of Clinical Endocrinology and Metabolism 65 (5): 1067–71. Nov 1987. doi:10.1210/jcem-65-5-1067. PMID 3667877.
- "Amniotic fluid concentrations of secreted pregnancy-associated endometrial alpha 1- and alpha 2-globulins (alpha 1- and alpha 2-PEG)". British Journal of Obstetrics and Gynaecology 93 (9): 909–15. Sep 1986. doi:10.1111/j.1471-0528.1986.tb08007.x. PMID 3768286.
- "A progestagen-dependent endometrial protein in human amniotic fluid". Journal of Reproduction and Fertility 60 (2): 317–21. Nov 1980. doi:10.1530/jrf.0.0600317. PMID 6776278.
- "Localization of alpha-uterine protein in human endometrium". Journal of Reproduction and Fertility 65 (2): 447–50. Jul 1982. doi:10.1530/jrf.0.0650447. PMID 7047733.
