Biology:Porphobilinogen deaminase
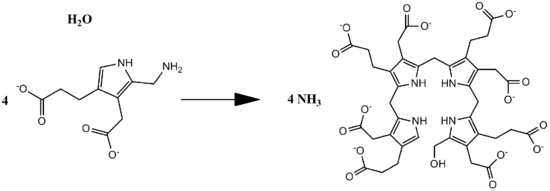
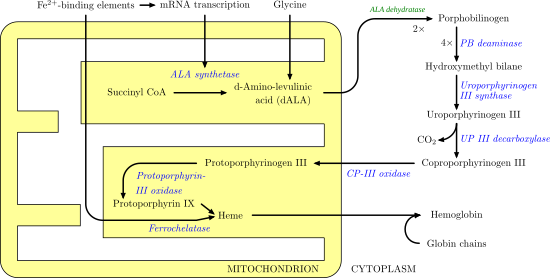
Porphobilinogen deaminase (hydroxymethylbilane synthase, or uroporphyrinogen I synthase) is an enzyme (EC 2.5.1.61) that in humans is encoded by the HMBS gene. Porphobilinogen deaminase is involved in the third step of the heme biosynthetic pathway. It catalyzes the head to tail condensation of four porphobilinogen molecules into the linear hydroxymethylbilane while releasing four ammonia molecules:
- 4 porphobilinogen + H2O [math]\displaystyle{ \rightleftharpoons }[/math] hydroxymethylbilane + 4 NH3
Structure and function
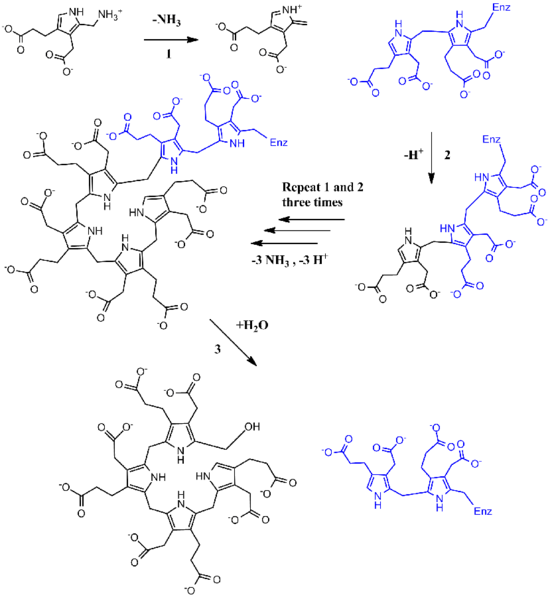
Functionally, porphobilinogen deaminase catalyzes the loss of ammonia from the porphobilinogen monomer (deamination) and its subsequent polymerization to a linear tetrapyrrole, which is released as hydroxymethylbilane:
The structure of 40-42 kDa porphobilinogen deaminase, which is highly conserved amongst organisms, consists of three domains.[1][2] Domains 1 and 2 are structurally very similar: each consisting of five beta-sheets and three alpha helices in humans.[3] Domain 3 is positioned between the other two and has a flattened beta-sheet geometry. A dipyrrole, a cofactor of this enzyme consisting of two condensed porphobilinogen molecules, is covalently attached to domain 3 and extends into the active site, the cleft between domains 1 and 2.[4] Several positively charged arginine residues, positioned to face the active site from domains 1 and 2, have been shown to stabilize the carboxylate functionalities on the incoming porphobilinogen as well as the growing pyrrole chain. These structural features presumably favor the formation of the final hydroxymethylbilane product.[5] Porphobilinogen deaminase usually exists in dimer units in the cytoplasm of the cell.
Reaction mechanism
The first step is believed to involve an E1 elimination of ammonia from porphobilinogen, generating a carbocation intermediate (1).[6] This intermediate is then attacked by the dipyrrole cofactor of porphobilinogen deaminase, which after losing a proton yields a trimer covalently bound to the enzyme (2). This intermediate is then open to further reaction with porphobilinogen (1 and 2 repeated three more times). Once a hexamer is formed, hydrolysis allows hydroxymethylbilane to be released, as well as cofactor regeneration (3).[7][8]
Pathology
The most well-known health issue involving porphobilinogen deaminase is acute intermittent porphyria, an autosomal dominant genetic disorder where insufficient hydroxymethylbilane is produced, leading to a build-up of porphobilinogen in the cytoplasm. This is caused by a gene mutation that, in 90% of cases, causes decreased amounts of enzyme. However, mutations where less-active enzymes and/or different isoforms have been described.[9][10][11] At least 115 disease-causing mutations in this gene have been discovered.[12]
References
- ↑ "Porphobilinogen deaminase in human erythrocytes: purification of two forms with apparent molecular weights of 40 kDa and 42 kDa". Scand. J. Clin. Lab. Invest. 49 (7): 677–84. November 1989. doi:10.3109/00365518909091544. PMID 2609111.
- ↑ "Structure of porphobilinogen deaminase reveals a flexible multidomain polymerase with a single catalytic site". Nature 359 (6390): 33–9. September 1992. doi:10.1038/359033a0. PMID 1522882. Bibcode: 1992Natur.359...33L.
- ↑ "Structure of human porphobilinogen deaminase at 2.8 Å: the molecular basis of acute intermittent porphyria". Biochem. J. 420 (1): 17–25. May 2009. doi:10.1042/BJ20082077. PMID 19207107. https://hal.archives-ouvertes.fr/hal-00479112/file/PEER_stage2_10.1042%252FBJ20082077.pdf.
- ↑ "Evidence for a dipyrromethane cofactor at the catalytic site of E. coli porphobilinogen deaminase". FEBS Lett. 225 (1–2): 87–92. December 1987. doi:10.1016/0014-5793(87)81136-5. PMID 3079571.
- ↑ "Studies on the mechanism of hydroxymethylbilane synthase concerning the role of arginine residues in substrate binding". Biochem. J. 275 (2): 447–52. April 1991. doi:10.1042/bj2750447. PMID 2025226.
- ↑ "On the mechanism of porphobilinogen deaminase. Design, synthesis, and enzymatic reactions of novel porphobilinogen analogs.". Tetrahedron 48 (23): 4687–4712. June 1992. doi:10.1016/S0040-4020(01)81567-2.
- ↑ Battersby AR (December 2000). "Tetrapyrroles: the pigments of life". Nat Prod Rep 17 (6): 507–26. doi:10.1039/b002635m. PMID 11152419.
- ↑ Leeper FJ (April 1989). "The biosynthesis of porphyrins, chlorophylls, and vitamin B12". Nat Prod Rep 6 (2): 171–203. doi:10.1039/NP9890600171. PMID 2664584.
- ↑ "Entrez Gene: HMBS hydroxymethylbilane synthase". https://www.ncbi.nlm.nih.gov/sites/entrez?Db=gene&Cmd=ShowDetailView&TermToSearch=3145.
- ↑ "A point mutation G----A in exon 12 of the porphobilinogen deaminase gene results in exon skipping and is responsible for acute intermittent porphyria". Nucleic Acids Res. 17 (16): 6637–49. August 1989. doi:10.1093/nar/17.16.6637. PMID 2789372.
- ↑ "Molecular basis of acute intermittent porphyria: mutations and polymorphisms in the human hydroxymethylbilane synthase gene". Hum. Mutat. 4 (4): 243–52. 1994. doi:10.1002/humu.1380040403. PMID 7866402.
- ↑ "Refinement of evolutionary medicine predictions based on clinical evidence for the manifestations of Mendelian diseases". Scientific Reports 9 (1): 18577. December 2019. doi:10.1038/s41598-019-54976-4. PMID 31819097. Bibcode: 2019NatSR...918577S.
Further reading
- "Porphobilinogen deaminase gene structure and molecular defects.". J. Bioenerg. Biomembr. 27 (2): 197–205. 1995. doi:10.1007/BF02110034. PMID 7592566.
- "Molecular basis of acute intermittent porphyria: mutations and polymorphisms in the human hydroxymethylbilane synthase gene.". Hum. Mutat. 4 (4): 243–52. 1995. doi:10.1002/humu.1380040403. PMID 7866402.
- "Time-resolved and static-ensemble structural chemistry of hydroxymethylbilane synthase.". Faraday Discussions 122: 131–44; discussion 171–90. 2003. doi:10.1039/b201331b. PMID 12555854. Bibcode: 2003FaDi..122..131H.
- "Homozygous acute intermittent porphyria in a 7-year-old boy with massive excretions of porphyrins and porphyrin precursors.". J. Inherit. Metab. Dis. 27 (1): 19–27. 2004. doi:10.1023/B:BOLI.0000016613.75677.05. PMID 14970743.
- Kauppinen R (2004). "Molecular diagnostics of acute intermittent porphyria.". Expert Rev. Mol. Diagn. 4 (2): 243–9. doi:10.1586/14737159.4.2.243. PMID 14995910.
- "May 2006 update in porphobilinogen deaminase gene polymorphisms and mutations causing acute intermittent porphyria: comparison with the situation in Slavic population.". Physiological Research 55 (Suppl 2): S119–36. 2007. PMID 17298216.
- "CRIM-positive mutations of acute intermittent porphyria in Finland.". Hum. Mutat. 1 (5): 392–6. 1993. doi:10.1002/humu.1380010508. PMID 1301948.
- "Detection of seven point mutations in the porphobilinogen deaminase gene in patients with acute intermittent porphyria, by direct sequencing of in vitro amplified cDNA.". Hum. Genet. 90 (1–2): 12–6. 1992. doi:10.1007/BF00210738. PMID 1427766.
- "High frequency of mutations in exon 10 of the porphobilinogen deaminase gene in patients with a CRIM-positive subtype of acute intermittent porphyria". Am. J. Hum. Genet. 51 (3): 660–5. 1992. PMID 1496994.
- "Molecular heterogeneity of acute intermittent porphyria: identification of four additional mutations resulting in the CRIM-negative subtype of the disease". Am. J. Hum. Genet. 49 (2): 421–8. 1991. PMID 1714233.
- "Assignment of human porphobilinogen deaminase to 11q24.1----q24.2 by in situ hybridization and gene dosage studies". Cytogenet. Cell Genet. 57 (2–3): 105–8. 1991. doi:10.1159/000133123. PMID 1914516.
- "Identification of the most common mutation within the porphobilinogen deaminase gene in Swedish patients with acute intermittent porphyria". Proc. Natl. Acad. Sci. U.S.A. 88 (23): 10912–5. 1992. doi:10.1073/pnas.88.23.10912. PMID 1961762.
- "A physical linkage group in human chromosome band 11q23 covering a region implicated in leukocyte neoplasia". Genomics 8 (3): 447–53. 1991. doi:10.1016/0888-7543(90)90030-X. PMID 1981047.
- "Acute intermittent porphyria caused by a C----T mutation that produces a stop codon in the porphobilinogen deaminase gene". Hum. Genet. 85 (6): 631–4. 1990. doi:10.1007/BF00193588. PMID 2227955.
- "Two different point G to A mutations in exon 10 of the porphobilinogen deaminase gene are responsible for acute intermittent porphyria". J. Clin. Invest. 86 (5): 1511–6. 1990. doi:10.1172/JCI114869. PMID 2243128.
- "Molecular cloning and complete primary sequence of human erythrocyte porphobilinogen deaminase". Nucleic Acids Res. 14 (15): 5955–68. 1986. doi:10.1093/nar/14.15.5955. PMID 2875434.
- "A 5' splice-region G----C mutation in exon 1 of the human beta-globin gene inhibits pre-mRNA splicing: a mechanism for beta+-thalassemia". Proc. Natl. Acad. Sci. U.S.A. 86 (3): 1041–5. 1989. doi:10.1073/pnas.86.3.1041. PMID 2915972. Bibcode: 1989PNAS...86.1041V.
External links
- Overview of all the structural information available in the PDB for UniProt: P08397 (Porphobilinogen deaminase) at the PDBe-KB.
 |