Biology:Farnesyl-diphosphate farnesyltransferase
| Squalene synthase | |||||||||
|---|---|---|---|---|---|---|---|---|---|
 | |||||||||
| Identifiers | |||||||||
| EC number | 2.5.1.21 | ||||||||
| CAS number | 9077-14-9 | ||||||||
| Databases | |||||||||
| IntEnz | IntEnz view | ||||||||
| BRENDA | BRENDA entry | ||||||||
| ExPASy | NiceZyme view | ||||||||
| KEGG | KEGG entry | ||||||||
| MetaCyc | metabolic pathway | ||||||||
| PRIAM | profile | ||||||||
| PDB structures | RCSB PDB PDBe PDBsum | ||||||||
| Gene Ontology | AmiGO / QuickGO | ||||||||
| |||||||||
| farnesyl-diphosphate farnesyltransferase 1 | |
|---|---|
| Identifiers | |
| Symbol | FDFT1 |
| NCBI gene | 2222 |
| HGNC | 3629 |
| OMIM | 184420 |
| RefSeq | NM_004462 |
| UniProt | P37268 |
| Other data | |
| EC number | 2.5.1.21 |
| Locus | Chr. 8 p23.1-p22 |
Squalene synthase (SQS) or farnesyl-diphosphate:farnesyl-diphosphate farnesyl transferase is an enzyme localized to the membrane of the endoplasmic reticulum. SQS participates in the isoprenoid biosynthetic pathway, catalyzing a two-step reaction in which two identical molecules of farnesyl pyrophosphate (FPP) are converted into squalene, with the consumption of NADPH.[2] Catalysis by SQS is the first committed step in sterol synthesis, since the squalene produced is converted exclusively into various sterols, such as cholesterol, via a complex, multi-step pathway. SQS belongs to squalene/phytoene synthase family of proteins.
Diversity
Squalene synthase has been characterized in animals, plants, and yeast.[3] In terms of structure and mechanics, squalene synthase closely resembles phytoene synthase (PHS), another prenyltransferase. PHS serves a similar role to SQS in plants and bacteria, catalyzing the synthesis of phytoene, a precursor of carotenoid compounds.[4]
Structure
Squalene synthase (SQS) is localized exclusively to the membrane of the endoplasmic reticulum (ER).[5] SQS is anchored to the membrane by a short C-terminal membrane-spanning domain.[6] The N-terminal catalytic domain of the enzyme protrudes into the cytosol, where the soluble substrates are bound.[2] Mammalian forms of SQS are approximately 47kDa and consist of ~416 amino acids. The crystal structure of human SQS was determined in 2000, and revealed that the protein was composed entirely of α-helices. The enzyme is folded into a single domain, characterized by a large central channel. The active sites of both of the two half-reactions catalyzed by SQS are located within this channel. One end of the channel is open to the cytosol, whereas the other end forms a hydrophobic pocket.[5] SQS contains two conserved aspartate-rich sequences, which are believed to participate directly in the catalytic mechanism.[7] These aspartate-rich motifs are one of several conserved structural features in class I isoprenoid biosynthetic enzymes, although these enzymes do not share sequence homology.[5]

Mechanism
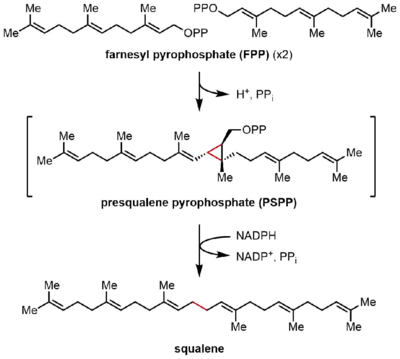
Squalene synthase (SQS) catalyzes the reductive dimerization of farnesyl pyrophosphate (FPP), in which two identical molecules of FPP are converted into one molecule of squalene. The reaction occurs in two steps, proceeding through the intermediate presqualene pyrophosphate (PSPP). FPP is a soluble allylic compound containing 15 carbon atoms (C15), whereas squalene is an insoluble, C30 isoprenoid.[2][4] This reaction is a head-to-head terpene synthesis, because the two FPP molecules are both joined at the C4 position and form a 1-1' linkage. This stands in contrast to the 1'-4 linkages that are much more common in isoprene biosynthesis than 4-4' linkages.[8][9] The reaction mechanism of SQS requires a divalent cation, often Mg2+, to facilitate binding of the pyrophosphate groups on FPP.[10]
FPP condensation
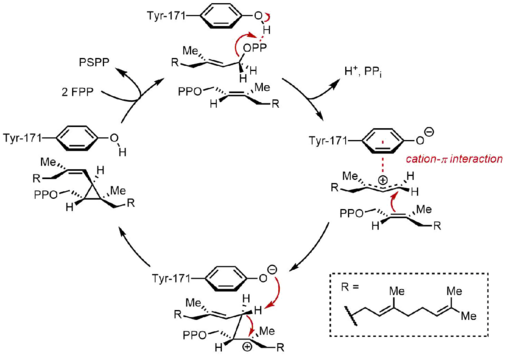
In the first half-reaction, two identical molecules of farnesyl pyrophosphate (FPP) are bound to squalene synthase (SQS) in a sequential manner. The FPP molecules bind to distinct regions of the enzyme, and with different binding affinities.[11] Starting at the top of the catalytic cycle below, the reaction begins with the ionization of FPP to generate an allylic carbocation. A tyrosine residue (Tyr-171) plays a critical role in this step by serving as a proton donor to facilitate abstraction of pyrophosphate. Moreover, the resulting phenolate anion can stabilize the resulting carbocation through cation-π interactions, which would be particularly strong due to the highly electron-rich nature of the phenolate anion. The allylic cation generated is then attacked by the olefin of a second molecule of FPP, affording a tertiary carbocation. The phenolate anion generated previously then serves as a base to abstract a proton from this adduct to form a cyclopropane product, presqualene pyrophosphate (PSPP). The PSPP created remains associated with SQS for the second reaction.[5][10] The importance of a tyrosine residue in this process was demonstrated by mutagenesis studies with rat SQS (rSQS),[7] and by the fact that Tyr-171 is conserved in all known SQSs (and PHSs).[2] In rSQS, Tyr-171 was converted to aromatic residues Phe and Trp, as well as hydroxyl-containing residue Ser. None of these mutants were able to convert FPP to PSPP or squalene, demonstrating that aromatic rings or alcohols alone are insufficient for converting FPP to PSPP.
PSPP rearrangement and reduction
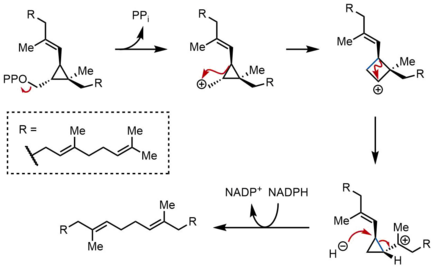
In the second half-reaction of SQS, presqualene pyrophosphate (PSPP) moves to a second reaction site within SQS. Keeping PSPP in the central channel of SQS is thought to protect the reactive intermediate from reacting with water.[5] From PSPP, squalene is formed by a series of carbocation rearrangements.[12][13] The process begins with ionization of pyrophosphate, giving a cyclopropylcarbinyl cation. The cation rearranges by a 1,2-migration of a cyclopropane C–C bond to the carbocation, forming the bond shown in blue to give a cyclobutyl carbocation. Subsequently, a second 1,2-migration occurs to form another cyclopropylcarbinyl cation, with the cation resting on a tertiary carbon. This resulting carbocation is then ring-opened by a hydride delivered by NADPH, giving squalene, which is then released by SQS into the membrane of the endoplasmic reticulum.[2]
While cyclopropylcarbinyl-cyclopropylcarbinyl rearrangements can proceed through discrete cyclobutyl cation intermediates, the supposed cyclobutyl cation could not be trapped in model studies. Thus, the cyclobutyl cation may actually be a transition state between the two cyclopropylcarbinyl cations, rather than a discrete intermediate. The stereochemistry of the intermediates and the olefin geometry in the final product is dictated by the suprafacial nature of the 1,2-shifts and stereoelectronic requirements. While other mechanisms have been proposed, the mechanism shown above is supported by isolation of rillingol, which is the alcohol formed from trapping the second cyclopropylcarbinyl cation with water.
Regulation

FPP is an important metabolic intermediate in the mevalonate pathway that represents a major branch point in terpenoid pathways.[2][14] FPP is used to form several important classes of compounds in addition to sterols (via squalene), including ubiquinone[15] and dolichols.[16] SQS catalyzes the first committed step in sterol biosynthesis from FPP, and is therefore important for controlling the flux towards sterol vs. non-sterol products. The activity of SQS is intimately related to the activity of HMG-CoA reductase, which catalyzes the rate-limiting step of the mevalonate pathway. High levels of LDL-derived cholesterol inhibit HMG-CoA reductase activity significantly, since mevalonate is no longer needed for sterol production. However, residual HMG-CoA reductase activity is observed even with very high LDL levels, such that FPP can be made for forming non-sterol products essential for cell growth.[17] To prevent this residual FPP from being used for sterol synthesis when sterols are abundant, SQS activity declines significantly when LDL levels are high.[18] This suppression of SQS activity is better thought of as a flux control mechanism, rather than a way to regulate cholesterol levels. This is since HMG-CoA reductase is the more significant control factor for regulating cholesterol synthesis (its activity is 98% inhibited when LDL levels are high).[17]
Regulation by sterols
SQS regulation occurs primarily at the level of SQS gene transcription.[2] The sterol regulatory element binding protein (SREBP) class of transcription factors is central to regulating genes involved in cholesterol homeostasis, and is important for controlling levels of SQS transcription. When sterol levels are low, an inactive form of SREBP is cleaved to form the active transcription factor, which moves to the nucleus to induce transcription of the SQS gene. Of the three known SREBP transcription factors, only SREBP-1a and SREBP-2 activate SQS gene transcription in transgenic mouse livers.[19][20] In cultured HepG2 cells, SREBP-1a appears more important than SREBP-2 in controlling activation of the SQS promoter.[21] However, SQS promoters have been shown to respond differently to SREBP-1a and SREBP-2 in different experimental systems.
Aside from SREBPs, accessory transcription factors are needed for maximal activation of the SQS promoter. Promoter studies using luciferase reporter gene assays revealed that the Sp1, and NF-Y and/or CREB transcription factors are also important for SQS promoter activation. NF-Y and/or CREB are required for SREBP-1a to fully activate the SQS promoter, although Sp1 is also needed for SREBP-2 to do so.
Interactive pathway map
Biological Function
Squalene synthase (SQS) is an enzyme participating in the isoprenoid biosynthetic pathway. SQS synthase catalyzes the branching point between sterol and nonsterol biosynthesis, and commits farnesyl pyrophosphate (FPP) exclusively to production of sterols.[2] An important sterol produced by this pathway is cholesterol, which is used in cell membranes and for the synthesis of hormones.[22] SQS competes with several other enzymes for use of FPP, since it is a precursor for a variety of terpenoids. Decreases in SQS activity limit flux of FPP to the sterol pathway, and increase the production of nonsterol products. Important nonsterol products include ubiquinone, dolichols, heme A, and farnesylated proteins [23]
Development of squalene synthase knockout mice has demonstrated that loss of squalene synthase is lethal, and that the enzyme is essential for development of the central nervous system.[24]
Disease Relevance
Squalene synthase is a target for the regulation of cholesterol levels. Increased expression of SQS has been shown to elevate cholesterol levels in mice.[24] Therefore, inhibitors of SQS are of great interest in the treatment of hypercholesterolemia and prevention of coronary heart disease (CHD).[25] It has also been suggested that variants in this enzyme may be part of a genetic association with hypercholesterolemia.[26]
Squalene synthase inhibitors
Squalene synthase inhibitors have been shown to decrease cholesterol synthesis, as well as to decrease plasma triglyceride levels.[22][27] SQS inhibitors may provide an alternative to HMG-CoA reductase inhibitors (statins), which have problematic side effects for some patients.[28] Squalene synthase inhibitors that have been investigated for use in the prevention of cardiovascular disease include lapaquistat (TAK-475), zaragozic acid, and RPR 107393.[29][30] Despite reaching phase II clinical trials, lapaquistat was discontinued by 2008.[31][32]
Squalene synthase homolog inhibition in Staphylococcus aureus is currently being investigated as a virulence factor-based antibacterial therapy.[33]
References
- ↑ "Discovery of a new 2-aminobenzhydrol template for highly potent squalene synthase inhibitors". Bioorg. Med. Chem. 19 (6): 1930–49. March 2011. doi:10.1016/j.bmc.2011.01.065. PMID 21353782.
- ↑ 2.0 2.1 2.2 2.3 2.4 2.5 2.6 2.7 "Structure and regulation of mammalian squalene synthase". Biochim. Biophys. Acta 1529 (1–3): 49–62. December 2000. doi:10.1016/S1388-1981(00)00137-2. PMID 11111077.
- ↑ "Cloning, expression, and characterization of cDNAs encoding Arabidopsis thaliana squalene synthase". Proc. Natl. Acad. Sci. U.S.A. 92 (6): 2328–32. March 1995. doi:10.1073/pnas.92.6.2328. PMID 7892265. Bibcode: 1995PNAS...92.2328N.
- ↑ 4.0 4.1 Squalene synthase: structure and regulation. Progress in Nucleic Acid Research and Molecular Biology. 65. 2001. 157–95. doi:10.1016/S0079-6603(00)65005-5. ISBN 9780125400657.
- ↑ 5.0 5.1 5.2 5.3 5.4 "Crystal structure of human squalene synthase. A key enzyme in cholesterol biosynthesis". J. Biol. Chem. 275 (39): 30610–7. September 2000. doi:10.1074/jbc.M004132200. PMID 10896663.
- ↑ "Molecular cloning and characterization of the yeast gene for squalene synthetase". Proc. Natl. Acad. Sci. U.S.A. 88 (14): 6038–42. July 1991. doi:10.1073/pnas.88.14.6038. PMID 2068081. Bibcode: 1991PNAS...88.6038J.
- ↑ 7.0 7.1 "Function-structure studies and identification of three enzyme domains involved in the catalytic activity in rat hepatic squalene synthase". J. Biol. Chem. 273 (20): 12515–25. May 1998. doi:10.1074/jbc.273.20.12515. PMID 9575210.
- ↑ Poulter CD (1990). "Biosynthesis of non-head-to-tail terpenes. Formation of 1'-1 and 1'-3 linkages". Accounts of Chemical Research 23 (3): 70–77. doi:10.1021/ar00171a003.
- ↑ "Mechanism of action and inhibition of dehydrosqualene synthase". Proc. Natl. Acad. Sci. U.S.A. 107 (50): 21337–42. December 2010. doi:10.1073/pnas.1010907107. PMID 21098670. Bibcode: 2010PNAS..10721337L.
- ↑ 10.0 10.1 "Squalene synthetase. 3. Mechanism of the reaction". J. Biol. Chem. 248 (5): 1856–67. March 1973. doi:10.1016/S0021-9258(19)44269-5. PMID 4348553.
- ↑ "Yeast squalene synthase. A mechanism for addition of substrates and activation by NADPH". J. Biol. Chem. 269 (15): 11201–7. April 1994. doi:10.1016/S0021-9258(19)78111-3. PMID 8157649.
- ↑ Blagg, Brian S. J.; Jarstfer, Michael B.; Rogers, Daniel H.; Poulter, C. Dale (2002-07-04). "Recombinant Squalene Synthase. A Mechanism for the Rearrangement of Presqualene Diphosphate to Squalene" (in en). Journal of the American Chemical Society 124 (30): 8846–8853. doi:10.1021/ja020411a. PMID 12137537.
- ↑ Jarstfer, Michael B.; Blagg, Brian S. J.; Rogers, Daniel H.; Poulter, C. Dale (1996-12-25). "Biosynthesis of Squalene. Evidence for a Tertiary Cyclopropylcarbinyl Cationic Intermediate in the Rearrangement of Presqualene Diphosphate to Squalene" (in en). Journal of the American Chemical Society 118 (51): 13089–13090. doi:10.1021/ja963308s.
- ↑ Brown, Michael S.; Goldstein, Joseph L. (1980). "Multivalent feedback regulation of HMG CoA reductase, a control mechanism coordinating isoprenoid synthesis and cell growth". J. Lipid Res. 21 (5): 505–517. doi:10.1016/S0022-2275(20)42221-7. PMID 6995544.
- ↑ Olson, Robert E. (1967-01-01). Robert S. Harris, Ira G. Wool, John A. Loraine, G. F. Marrian and Kenneth V. Thimann. ed. "Biosynthesis of Ubiquinones in Animals*". Vitamins & Hormones 24: 551–574. doi:10.1016/s0083-6729(08)60221-6. ISBN 9780127098241. PMID 5340877.
- ↑ Gough, Doreen P.; Hemming, F. W. (1970-06-01). "The characterization and stereochemistry of biosynthesis of dolichols in rat liver". Biochemical Journal 118 (1): 163–166. doi:10.1042/bj1180163. ISSN 0264-6021. PMID 4319540.
- ↑ 17.0 17.1 Faust, Jerry R.; Goldstein, Joseph L.; Brown, Michael S. (1979-01-01). "Synthesis of ubiquinone and cholesterol in human fibroblasts: Regulation of a branched pathway". Archives of Biochemistry and Biophysics 192 (1): 86–99. doi:10.1016/0003-9861(79)90074-2. PMID 219777.
- ↑ Faust, Jerry R.; Goldstein, Joseph L.; Brown, Michael S. (1979-10-01). "Squalene synthetase activity in human fibroblasts: Regulation via the low density lipoprotein receptor". Proceedings of the National Academy of Sciences of the United States of America 76 (10): 5018–5022. doi:10.1073/pnas.76.10.5018. ISSN 0027-8424. PMID 228272. Bibcode: 1979PNAS...76.5018F.
- ↑ Guan, G.; Jiang, G.; Koch, R. L.; Shechter, I. (1995-09-15). "Molecular cloning and functional analysis of the promoter of the human squalene synthase gene". The Journal of Biological Chemistry 270 (37): 21958–21965. doi:10.1074/jbc.270.37.21958. ISSN 0021-9258. PMID 7665618.
- ↑ Guan, Guimin; Dai, Pei-Hua; Osborne, Timothy F.; Kim, Jae B.; Shechter, Ishaiahu (1997-04-11). "Multiple Sequence Elements are Involved in the Transcriptional Regulation of the Human Squalene Synthase Gene" (in en). Journal of Biological Chemistry 272 (15): 10295–10302. doi:10.1074/jbc.272.15.10295. ISSN 0021-9258. PMID 9092581.
- ↑ Guan, G.; Dai, P.; Shechter, I. (1998-05-15). "Differential transcriptional regulation of the human squalene synthase gene by sterol regulatory element-binding proteins (SREBP) 1a and 2 and involvement of 5' DNA sequence elements in the regulation". The Journal of Biological Chemistry 273 (20): 12526–12535. doi:10.1074/jbc.273.20.12526. ISSN 0021-9258. PMID 9575211.
- ↑ 22.0 22.1 "Squalene synthase inhibitors: An update on the search for new antihyperlipidemic and antiatherosclerotic agents". Curr. Med. Chem. 18 (29): 4418–39. 2011. doi:10.2174/092986711797287557. PMID 21864285.
- ↑ "Redirection of flux through the FPP branch-point in Saccharomyces cerevisiae by down-regulating squalene synthase". Biotechnol. Bioeng. 100 (2): 371–8. June 2008. doi:10.1002/bit.21766. PMID 18175359.
- ↑ 24.0 24.1 "Increased cholesterol biosynthesis and hypercholesterolemia in mice overexpressing squalene synthase in the liver". J. Lipid Res. 47 (9): 1950–8. September 2006. doi:10.1194/jlr.M600224-JLR200. PMID 16741291.
- ↑ Davidson MH (January 2007). "Squalene synthase inhibition: a novel target for the management of dyslipidemia". Curr Atheroscler Rep 9 (1): 78–80. doi:10.1007/BF02693932. PMID 17169251.
- ↑ "Squalene synthase: a critical enzyme in the cholesterol biosynthesis pathway". Clin. Genet. 75 (1): 19–29. January 2009. doi:10.1111/j.1399-0004.2008.01099.x. PMID 19054015.
- ↑ "Squalene synthase inhibitors reduce plasma triglyceride through a low-density lipoprotein receptor-independent mechanism". Eur. J. Pharmacol. 431 (3): 345–52. November 2001. doi:10.1016/S0014-2999(01)01450-9. PMID 11730728.
- ↑ "Pharmacologic inhibition of squalene synthase and other downstream enzymes of the cholesterol synthesis pathway: a new therapeutic approach to treatment of hypercholesterolemia". Cardiol Rev 17 (2): 70–6. 2009. doi:10.1097/CRD.0b013e3181885905. PMID 19367148.
- ↑ "Squalene synthase inhibitors : clinical pharmacology and cholesterol-lowering potential". Drugs 67 (1): 11–6. 2007. doi:10.2165/00003495-200767010-00002. PMID 17209661.
- ↑ "RPR 107393, a potent squalene synthase inhibitor and orally effective cholesterol-lowering agent: comparison with inhibitors of HMG-CoA reductase". J. Pharmacol. Exp. Ther. 281 (2): 746–52. May 1997. PMID 9152381.
- ↑ Gibbs, Edwina (29 October 2007). "UPDATE 2-US FDA tells Takeda to stop some TAK-475 trials". Reuters. https://www.reuters.com/article/takeda-cholesterol-fda-idUST17428120071029.
- ↑ "Discontinuation of Development of TAK-475, A Compound for Treatment of Hypercholesterolemia". Takeda Pharmaceutical Company Limited. 28 March 2008. http://www.takeda.com/news/2008/20080328_3603.html.
- ↑ "A cholesterol biosynthesis inhibitor blocks Staphylococcus aureus virulence". Science 319 (5868): 1391–4. March 2008. doi:10.1126/science.1153018. PMID 18276850. PMC 2747771. Bibcode: 2008Sci...319.1391L. http://ntur.lib.ntu.edu.tw/bitstream/246246/163379/1/40.pdf.
External links
- Farnesyl-Diphosphate+Farnesyltransferase at the US National Library of Medicine Medical Subject Headings (MeSH)
 |