Biology:Sda protein domain
| Sda | |||||||||
|---|---|---|---|---|---|---|---|---|---|
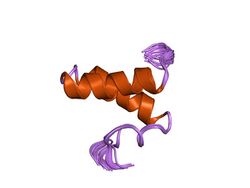 structure of the sda antikinase | |||||||||
| Identifiers | |||||||||
| Symbol | Sda | ||||||||
| Pfam | PF08970 | ||||||||
| InterPro | IPR015064 | ||||||||
| |||||||||
In molecular biology, the protein domain Sda is short for suppressor of dnaA or otherwise known as sporulation inhibitor A. It is found only in bacteria. This protein domain is highly important to cell survival. When starved of nutrients, the cell is under extreme stress so undergoes a series of reactions to increase the chances of survival. One method is to form endospores which can withstand a large amount of environmental pressure.[1] Sda protein domain is a checkpoint which prevents the formation of spores. The Sda domain affects cell signalling. It prevents the cell communicating the stress that it is under, which is crucial if the cell is to survive.
Cell signalling
All cells communicate through cell signalling. The Sda protein domain inhibits the Histidine kinase signal transduction. This signal transduction mechanism is switched on when the cell is under stress, such as nutrient deprivation. It works through the kinase first autophosphorylating the Histidine residue and this triggers sporulation. Sda prevents this pathway from occurring by inhibiting the histidine kinase. More specifically, Sda can be considered an antikinase and binds to KinA. The Sda-KinA complex now physically blocks any phosphorylation of the Histidine Kinase residue.[2]
Function
These sporulation inhibitors are anti-kinases that bind to the histidine kinase KinA phosphotransfer protein domain and act as a molecular barricade that inhibit productive interaction between the ATP binding site and the phosphorylatable KinA His residue. This results in the inhibition of sporulation through the prevention of phosphorylation of spo0A, a transcription factor.[2]
Structure
Members of this protein group contain two antiparallel alpha helices that are linked by an inter-helix loop to form a helical hairpin. These monomers associate to form a simple trimeric arrangement.[3]
References
- ↑ Burkholder WF; Kurtser I; Grossman AD (2001). "Replication initiation proteins regulate a developmental checkpoint in Bacillus subtilis.". Cell 104 (2): 269–79. doi:10.1016/s0092-8674(01)00211-2. PMID 11207367.
- ↑ 2.0 2.1 Rowland SL; Burkholder WF; Cunningham KA; Maciejewski MW; Grossman AD; King GF (2004). "Structure and mechanism of action of Sda, an inhibitor of the histidine kinases that regulate initiation of sporulation in Bacillus subtilis.". Mol Cell 13 (5): 689–701. doi:10.1016/S1097-2765(04)00084-X. PMID 15023339.
- ↑ "Structure of the sporulation histidine kinase inhibitor Sda from Bacillus subtilis and insights into its solution state.". Acta Crystallogr D 65 (Pt 6): 574–81. 2009. doi:10.1107/S090744490901169X. PMID 19465772. PMC 2725781. http://espace.library.uq.edu.au/view/UQ:180915/UQ180915_OA.pdf.
 |

