Biology:Sema domain
 Sema domain, immunoglobulin domain (Ig), short basic domain | |||||||||
| Identifiers | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Symbol | Sema | ||||||||
| Pfam | PF01403 | ||||||||
| InterPro | IPR001627 | ||||||||
| PROSITE | PDOC51004 | ||||||||
| SCOP2 | 1olz / SCOPe / SUPFAM | ||||||||
| Membranome | 71 | ||||||||
| |||||||||
The Sema domain is a structural domain of semaphorins, which are a large family of secreted and transmembrane proteins, some of which function as repellent signals during axon guidance. Sema domains also occur in the hepatocyte growth factor receptor (Uniprot: P08581), Plexin-A3 [1] (Uniprot: P51805) and in viral proteins.
CD100 (also called SEMA4D) is associated with PTPase and serine kinase activity. CD100 increases PMA, CD3 and CD2 induced T cell proliferation, increases CD45 induced T cell adhesion, induces B cell homotypic adhesion and down-regulates B cell expression of CD23.
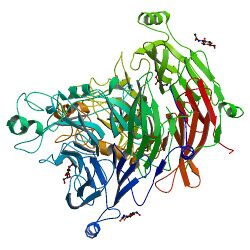
The Sema domain is characterised by a conserved set of cysteine residues, which form four disulfide bonds to stabilise the structure. The Sema domain fold is a variation of the beta propeller topology, with seven blades radially arranged around a central axis. Each blade contains a four- stranded (strands A to D) antiparallel beta sheet. The inner strand of each blade (A) lines the channel at the centre of the propeller, with strands B and C of the same repeat radiating outward, and strand D of the next repeat forming the outer edge of the blade. The large size of the Sema domain is not due to a single inserted domain but results from the presence of additional secondary structure elements inserted in most of the blades. The Sema domain uses a 'loop and hook' system to close the circle between the first and the last blades. The blades are constructed sequentially with an N-terminal beta- strand closing the circle by providing the outermost strand (D) of the seventh (C-terminal) blade. The beta-propeller is further stabilized by an extension of the N-terminus, providing an additional, fifth beta-strand on the outer edge of blade 6.[2][3][4]
Human proteins containing this domain
MET; MST1R; PLXNA1; PLXNA2; PLXNA3; PLXNA4; PLXNB1; PLXNB2; PLXNB3; PLXND1; SEMA3A; SEMA3B; SEMA3C; SEMA3D; SEMA3E; SEMA3F; SEMA3G; SEMA4A; SEMA4B; SEMA4C; SEMA4D; SEMA4F; SEMA4G; SEMA5A; SEMA5B; SEMA6A; SEMA6B; SEMA6C; SEMA6D; SEMA7A;
References
- ↑ "Plexin A is a neuronal semaphorin receptor that controls axon guidance". Cell 95 (7): 903–16. December 1998. doi:10.1016/S0092-8674(00)81715-8. PMID 9875845.
- ↑ "Structure of the semaphorin-3A receptor binding module". Neuron 39 (4): 589–98. August 2003. doi:10.1016/S0896-6273(03)00502-6. PMID 12925274.
- ↑ "The ligand-binding face of the semaphorins revealed by the high-resolution crystal structure of SEMA4D". Nature Structural Biology 10 (10): 843–8. October 2003. doi:10.1038/nsb977. PMID 12958590.
- ↑ "Crystal structure of the HGF beta-chain in complex with the Sema domain of the Met receptor". The EMBO Journal 23 (12): 2325–35. June 2004. doi:10.1038/sj.emboj.7600243. PMID 15167892.
 |

