Biology:Sonic hedgehog protein
 Generic protein structure example |
Sonic hedgehog protein (SHH) is encoded for by the SHH gene.[1] The protein is named after the character Sonic the Hedgehog.
This signaling molecule is key in regulating embryonic morphogenesis in all animals. SHH controls organogenesis and the organization of the central nervous system, limbs, digits and many other parts of the body. Sonic hedgehog is a morphogen that patterns the developing embryo using a concentration gradient characterized by the French flag model.[2] This model has a non-uniform distribution of SHH molecules which governs different cell fates according to concentration. Mutations in this gene can cause holoprosencephaly, a failure of splitting in the cerebral hemispheres,[3] as demonstrated in an experiment using SHH knock-out mice in which the forebrain midline failed to develop and instead only a single fused telencephalic vesicle resulted.[4]
Sonic hedgehog still plays a role in differentiation, proliferation, and maintenance of adult tissues. Abnormal activation of SHH signaling in adult tissues has been implicated in various types of cancers including breast, skin, brain, liver, gallbladder and many more.[5]
Discovery and naming
The hedgehog gene (hh) was first identified in the fruit fly Drosophila melanogaster in the classic Heidelberg screens of Christiane Nüsslein-Volhard and Eric Wieschaus, as published in 1980.[6] These screens, which led to the researchers winning a Nobel Prize in 1995 along with developmental geneticist Edward B. Lewis, identified genes that control the segmentation pattern of the Drosophila embryos. The hh loss of function mutant phenotype causes the embryos to be covered with denticles, i.e. small pointy projections resembling the spikes of a hedgehog. Investigations aimed at finding a hedgehog equivalent in vertebrates by Philip Ingham, Andrew P. McMahon and Clifford Tabin revealed three homologous genes.[7][8][9][10]
Two of these genes, desert hedgehog and Indian hedgehog, were named for species of hedgehogs, while sonic hedgehog was named after the video game character Sonic the Hedgehog.[11][12] The gene was named by Robert Riddle, a postdoctoral fellow at the Tabin Lab, after his wife Betsy Wilder came home with a magazine containing an advert for the game Sonic the Hedgehog.[13][14][15] In the zebrafish, two of the three vertebrate hh genes are duplicated: SHH a[16] and SHH b[17] (formerly described as tiggywinkle hedgehog, named for Mrs. Tiggy-Winkle, a character from Beatrix Potter's books for children) and ihha and ihhb[18] (formerly described as echidna hedgehog, named for the spiny anteater and not for the character Knuckles the Echidna in the Sonic franchise).
Function
Of the hh homologues, SHH has been found to have the most critical roles in development, acting as a morphogen involved in patterning many systems—including the anterior pituitary,[19] pallium of the brain,[20] spinal cord,[21] lungs,[22] teeth[23] and the thalamus by the zona limitans intrathalamica.[24][25] In vertebrates, the development of limbs and digits depends on the secretion of sonic hedgehog by the zone of polarizing activity, located on the posterior side of the embryonic limb bud.[9] Mutations in the human sonic hedgehog gene SHH cause holoprosencephaly type 3 HPE3, as a result of the loss of the ventral midline. The sonic hedgehog transcription pathway has also been linked to the formation of specific kinds of cancerous tumors, including the embryonic cerebellar tumor[26] and medulloblastoma,[27] as well as the progression of prostate cancer tumours.[28] For SHH to be expressed in the developing embryo limbs, a morphogen called fibroblast growth factors must be secreted from the apical ectodermal ridge.[29]
Sonic hedgehog has also been shown to act as an axonal guidance cue. It has been demonstrated that SHH attracts commissural axons at the ventral midline of the developing spinal cord.[30] Specifically, SHH attracts retinal ganglion cell (RGC) axons at low concentrations and repels them at higher concentrations.[31] The absence (non-expression) of SHH has been shown to control the growth of nascent hind limbs in cetaceans[32] (whales and dolphins).
The SHH gene is a member of the hedgehog gene family with five variations of DNA sequence alterations or splice variants.[33] SHH is located on chromosome seven and initiates the production of Sonic Hedgehog protein.[33] This protein sends short- and long-range signals to embryonic tissues to regulate development.[34] If the SHH gene is mutated or absent, the protein Sonic Hedgehog cannot do its job properly. Sonic hedgehog contributes to cell growth, cell specification and formation, structuring and organization of the body plan.[35] This protein functions as a vital morphogenic signaling molecule and plays an important role in the formation of many different structures in developing embryos.[35] The SHH gene affects several major organ systems, such as the nervous system, cardiovascular system, respiratory system and musculoskeletal system.[33][34] Mutations in the SHH gene can cause malformation of components of these systems, which can result in major problems in the developing embryo. The brain and eyes, for example, can be significantly impacted by mutations in this gene and cause disorders such as Microphthalmia and Holoprosencephaly.[35] Microphthalmia is a condition that affects the eyes, which results in small, underdeveloped tissues in one or both eyes.[35] This can lead to issues ranging from a coloboma to a single small eye to the absence of eyes altogether.[34] Holoprosencephaly is a condition most commonly caused by a mutation of the SHH gene that causes improper separation or turn of the left and right brain[36] and facial dysmorphia.[34][35] Many systems and structures rely heavily on proper expression of the SHH gene and subsequent sonic hedgehog protein, earning it the distinction of being an essential gene to development.
Patterning of the central nervous system
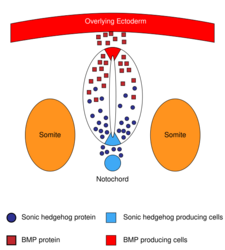
The sonic hedgehog (SHH) signaling molecule assumes various roles in patterning the central nervous system (CNS) during vertebrate development. One of the most characterized functions of SHH is its role in the induction of the floor plate and diverse ventral cell types within the neural tube.[37] The notochord—a structure derived from the axial mesoderm—produces SHH, which travels extracellularly to the ventral region of the neural tube and instructs those cells to form the floor plate.[38] Another view of floor plate induction hypothesizes that some precursor cells located in the notochord are inserted into the neural plate before its formation, later giving rise to the floor plate.[39]
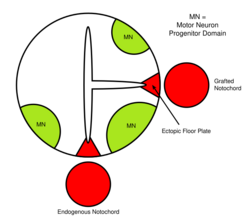
The neural tube itself is the initial groundwork of the vertebrate CNS, and the floor plate is a specialized structure, located at the ventral midpoint of the neural tube. Evidence supporting the notochord as the signaling center comes from studies in which a second notochord is implanted near a neural tube in vivo, leading to the formation of an ectopic floor plate within the neural tube.[40]
Sonic hedgehog is the secreted protein that mediates signaling activities of the notochord and floor plate.[41] Studies involving ectopic expression of SHH in vitro[42] and in vivo[43] result in floor plate induction and differentiation of motor neuron and ventral interneurons. On the other hand, mice mutants for SHH lack ventral spinal cord characteristics.[44] In vitro blocking of SHH signaling using antibodies against it shows similar phenotypes.[43] SHH exerts its effects in a concentration-dependent manner,[45] so that a high concentration of SHH results in a local inhibition of cellular proliferation.[46] This inhibition causes the floor plate to become thin compared to the lateral regions of the neural tube. Lower concentration of SHH results in cellular proliferation and induction of various ventral neural cell types.[43] Once the floor plate is established, cells residing in this region will subsequently express SHH themselves,[46] generating a concentration gradient within the neural tube.
Although there is no direct evidence of a SHH gradient, there is indirect evidence via the visualization of Patched (Ptc) gene expression, which encodes for the ligand binding domain of the SHH receptor[47] throughout the ventral neural tube.[48] In vitro studies show that incremental two- and threefold changes in SHH concentration give rise to motor neuron and different interneuronal subtypes as found in the ventral spinal cord.[49] These incremental changes in vitro correspond to the distance of domains from the signaling tissue (notochord and floor plate) which subsequently differentiates into different neuronal subtypes as it occurs in vitro.[50] Graded SHH signaling is suggested to be mediated through the Gli family of proteins, which are vertebrate homologues of the Drosophila zinc-finger-containing transcription factor Cubitus interruptus (Ci). Ci is a crucial mediator of hedgehog (Hh) signaling in Drosophila.[51] In vertebrates, three different Gli proteins are present, viz. Gli1, Gli2 and Gli3, which are expressed in the neural tube.[52] Mice mutants for Gli1 show normal spinal cord development, suggesting that it is dispensable for mediating SHH activity.[53] However, Gli2 mutant mice show abnormalities in the ventral spinal cord, with severe defects in the floor plate and ventral-most interneurons (V3).[54] Gli3 antagonizes SHH function in a dose-dependent manner, promoting dorsal neuronal subtypes. SHH mutant phenotypes can be rescued in a SHH/Gli3 double mutant.[55] Gli proteins have a C-terminal activation domain and an N-terminal repressive domain.[52][56]
SHH is suggested to promote the activation function of Gli2 and inhibit repressive activity of Gli3. SHH also seems to promote the activation function of Gli3, but this activity is not strong enough.[55] The graded concentration of SHH gives rise to graded activity of Gli 2 and Gli3, which promote ventral and dorsal neuronal subtypes in the ventral spinal cord. Evidence from Gli3 and SHH/Gli3 mutants show that SHH primarily regulates the spatial restriction of progenitor domains rather than being inductive, as SHH/Gli3 mutants show intermixing of cell types.[55][57]
SHH also induces other proteins with which it interacts, and these interactions can influence the sensitivity of a cell towards SHH. Hedgehog-interacting protein (HHIP) is induced by SHH, which in turn attenuates its signaling activity.[58] Vitronectin is another protein that is induced by SHH; it acts as an obligate co-factor for SHH signaling in the neural tube.[59]
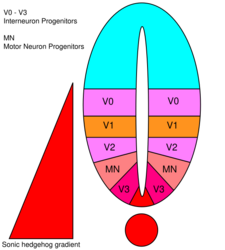
There are five distinct progenitor domains in the ventral neural tube: V3 interneurons, motor neurons (MN), V2, V1, and V0 interneurons (in ventral to dorsal order).[49] These different progenitor domains are established by "communication" between different classes of homeobox transcription factors. (See Trigeminal Nerve.) These transcription factors respond to SHH gradient concentration. Depending upon the nature of their interaction with SHH, they are classified into two groups—class I and class II—and are composed of members from the Pax, Nkx, Dbx and Irx families.[46] Class I proteins are repressed at different thresholds of SHH delineating ventral boundaries of progenitor domains, while class II proteins are activated at different thresholds of SHH delineating the dorsal limit of domains. Selective cross-repressive interactions between class I and class II proteins give rise to five cardinal ventral neuronal subtypes.[60]
It is important to note that SHH is not the only signaling molecule exerting an effect on the developing neural tube. Many other molecules, pathways and mechanisms are active (e.g., RA, FGF, BMP), and complex interactions between SHH and other molecules are possible. BMPs are suggested to play a critical role in determining the sensitivity of neural cell to SHH signaling. Evidence supporting this comes from studies using BMP inhibitors that ventralize the fate of the neural plate cell for a given SHH concentration.[61] On the other hand, mutation in BMP antagonists (e.g., noggin) produces severe defects in the ventral-most characteristics of the spinal cord, followed by ectopic expression of BMP in the ventral neural tube.[62] Interactions of SHH with Fgf and RA have not yet been studied in molecular detail.
Morphogenetic activity
The concentration- and time-dependent, cell-fate-determining activity of SHH in the ventral neural tube makes it a prime example of a morphogen. In vertebrates, SHH signaling in the ventral portion of the neural tube is most notably responsible for the induction of floor plate cells and motor neurons.[63] SHH emanates from the notochord and ventral floor plate of the developing neural tube to create a concentration gradient that spans the dorso-ventral axis and is antagonized by an inverse Wnt gradient, which specifies the dorsal spinal cord.[64][65] Higher concentrations of the SHH ligand are found in the most ventral aspects of the neural tube and notochord, while lower concentrations are found in the more dorsal regions of the neural tube.[64] The SHH concentration gradient has been visualized in the neural tube of mice engineered to express a SHH::GFP fusion protein to show this graded distribution of SHH during the time of ventral neural tube patterning.[66]
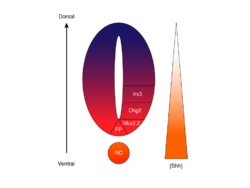
It is thought that the SHH gradient works to elicit multiple different cell fates by a concentration- and time-dependent mechanism that induces a variety of transcription factors in the ventral progenitor cells.[64][66] Each of the ventral progenitor domains expresses a highly individualized combination of transcription factors—Nkx2.2, Olig2, Nkx6.1, Nkx6.2, Dbx1, Dbx2, Irx3, Pax6, and Pax7—that is regulated by the SHH gradient. These transcription factors are induced sequentially along the SHH concentration gradient with respect to the amount and time of exposure to SHH ligand.[64] As each population of progenitor cells responds to the different levels of SHH protein, they begin to express a unique combination of transcription factors that leads to neuronal cell fate differentiation. This SHH-induced differential gene expression creates sharp boundaries between the discrete domains of transcription factor expression, which ultimately patterns the ventral neural tube.[64]
The spatial and temporal aspect of the progressive induction of genes and cell fates in the ventral neural tube is illustrated by the expression domains of two of the most well-characterized transcription factors, Olig2 and Nkx2.2.[64] Early in development, the cells at the ventral midline have only been exposed to a low concentration of SHH for a relatively short time and express the transcription factor Olig2.[64] The expression of Olig2 rapidly expands in a dorsal direction concomitantly with the continuous dorsal extension of the SHH gradient over time.[64] However, as the morphogenetic front of SHH ligand moves and begins to grow more concentrated, cells that are exposed to higher levels of the ligand respond by switching off Olig2 and turning on Nkx2.2,[64] creating a sharp boundary between the cells expressing the transcription factor Nkx2.2 ventral to the cells expressing Olig2. It is in this way that each of the domains of the six progenitor cell populations are thought to be successively patterned throughout the neural tube by the SHH concentration gradient.[64] Mutual inhibition between pairs of transcription factors expressed in neighboring domains contributes to the development of sharp boundaries; however, in some cases, inhibitory relationship has been found even between pairs of transcription factors from more distant domains. Particularly, NKX2-2 expressed in the V3 domain is reported to inhibit IRX3 expressed in V2 and more dorsal domains, although V3 and V2 are separated by a further domain termed MN.[67]
SHH expression in the frontonasal ectodermal zone (FEZ), which is a signaling center that is responsible for the patterned development of the upper jaw, regulates craniofacial development mediating through the miR-199 family in the FEZ. Specifically, SHH-dependent signals from the brain regulate genes of the miR-199 family with downregulations of the miR-199 genes increasing SHH expression and resulting in wider faces, while upregulations of the miR-199 genes decrease SHH expression resulting in narrow faces.[68]
Tooth development
SHH plays a very important role in organogenesis and, most importantly, craniofacial development. Being that SHH is a signaling molecule, it primarily works by diffusion along a concentration gradient, affecting cells in different manners. In early tooth development, SHH is released from the primary enamel knot—a signaling center—to provide positional information in both a lateral and planar signaling pattern in tooth development and regulation of tooth cusp growth.[69] SHH in particular is needed for growth of epithelial cervical loops, where the outer and inner epitheliums join and form a reservoir for dental stem cells. After the primary enamel knots are apoptosed, the secondary enamel knots are formed. The secondary enamel knots secrete SHH in combination with other signaling molecules to thicken the oral ectoderm and begin patterning the complex shapes of the crown of a tooth during differentiation and mineralization.[70] In a knockout gene model, absence of SHH is indicative of holoprosencephaly. However, SHH activates downstream molecules of Gli2 and Gli3. Mutant Gli2 and Gli3 embryos have abnormal development of incisors that are arrested in early tooth development as well as small molars.[71]
Lung development
Although SHH is most commonly associated with brain and limb digit development, it is also important in lung development.[72][73][74][75] Studies using qPCR and knockouts have demonstrated that SHH contributes to embryonic lung development. The mammalian lung branching occurs in the epithelium of the developing bronchi and lungs.[76][77] SHH expressed throughout the foregut endoderm (innermost of three germ layers) in the distal epithelium, where the embryonic lungs are developing.[74][77] This suggests that SHH is partially responsible for the branching of the lungs. Further evidence of SHH's role in lung branching has been seen with qPCR. SHH expression occurs in the developing lungs around embryonic day 11 and is strongly expressed in the buds of the fetal lungs but low in the developing bronchi.[74][77] Mice who are deficient in SHH can develop tracheoesophageal fistula (abnormal connection of the esophagus and trachea).[78][74] Additionally, a double (SHH-/- ) knockout mouse model exhibited poor lung development. The lungs of the SHH double knockout failed to undergo lobation and branching (i.e., the abnormal lungs only developed one branch, compared to an extensively branched phenotype of the wildtype).[74]
Potential regenerative function
Sonic hedgehog may play a role in mammalian hair cell regeneration. By modulating retinoblastoma protein activity in rat cochlea, sonic hedgehog allows mature hair cells that normally cannot return to a proliferative state to divide and differentiate. Retinoblastoma proteins suppress cell growth by preventing cells from returning to the cell cycle, thereby preventing proliferation. Inhibiting the activity of Rb seems to allow cells to divide. Therefore, sonic hedgehog—identified as an important regulator of Rb—may also prove to be an important feature in regrowing hair cells after damage.[79]
SHH is important for regulating dermal adipogenesis by hair follicle transit-amplifying cells (HF-TACs). Specifically, SHH induces dermal angiogenesis by acting directly on adipocyte precursors and promoting their proliferation through their expression of the peroxisome proliferator-activated receptor γ (Pparg) gene.[80]
Processing
SHH undergoes a series of processing steps before it is secreted from the cell. Newly synthesised SHH weighs 45 kDa and is referred to as the preproprotein. As a secreted protein, it contains a short signal sequence at its N-terminus, which is recognised by the signal recognition particle during the translocation into the endoplasmic reticulum (ER), the first step in protein secretion. Once translocation is complete, the signal sequence is removed by signal peptidase in the ER. There, SHH undergoes autoprocessing to generate a 20 kDa N-terminal signaling domain (SHH-N) and a 25 kDa C-terminal domain with no known signaling role.[81] The cleavage is catalysed by a protease within the C-terminal domain. During the reaction, a cholesterol molecule is added to the C-terminus of SHH-N.[82][83] Thus, the C-terminal domain acts as an intein and a cholesterol transferase. Another hydrophobic moiety, a palmitate, is added to the alpha-amine of N-terminal cysteine of SHH-N. This modification is required for efficient signaling, resulting in a 30-fold increase in potency over the non-palmitylated form and is carried out by a member of the membrane-bound O-acyltransferase family Protein-cysteine N-palmitoyltransferase HHAT.[84]
Robotnikinin
A potential inhibitor of the Hedgehog signaling pathway has been found and dubbed "Robotnikinin"—in honour of Sonic the Hedgehog's nemesis, Dr. Ivo "Eggman" Robotnik.[85]
Former controversy surrounding name
The gene has been linked to a condition known as holoprosencephaly, which can result in severe brain, skull and facial defects, causing a few clinicians and scientists to criticize the name on the grounds that it sounds too frivolous. It has been noted that mention of a mutation in a sonic hedgehog gene might not be well received in a discussion of a serious disorder with a patient or their family.[13][86][87] This controversy has largely died down, and the name is now generally seen as a humorous relic of the time before the rise of fast, cheap complete genome sequencing and standardized nomenclature.[88] The problem of the "inappropriateness" of the names of genes such as "Mothers against decapentaplegic", "Lunatic fringe", and "Sonic hedgehog" is largely avoided by using standardized abbreviations when speaking with patients and their families.[89]
Gallery
See also
- Pikachurin, a retinal protein named after Pikachu
- Zbtb7, an oncogene which was originally named "Pokémon"
References
- ↑ "Cloning, expression, and chromosomal location of SHH and IHH: two human homologues of the Drosophila segment polarity gene hedgehog". Genomics 28 (1): 44–51. July 1995. doi:10.1006/geno.1995.1104. PMID 7590746.
- ↑ "Getting the measure of positional information". PLOS Biology 7 (3): e81. March 2009. doi:10.1371/journal.pbio.1000081. PMID 19338391.
- ↑ "The mutational spectrum of the sonic hedgehog gene in holoprosencephaly: SHH mutations cause a significant proportion of autosomal dominant holoprosencephaly". Human Molecular Genetics 8 (13): 2479–2488. December 1999. doi:10.1093/hmg/8.13.2479. PMID 10556296.
- ↑ "Sonic hedgehog signaling in the development of the mouse hypothalamus". Frontiers in Neuroanatomy 8: 156. 2015-01-06. doi:10.3389/fnana.2014.00156. PMID 25610374.
- ↑ "Sonic Hedgehog Signaling in Organogenesis, Tumors, and Tumor Microenvironments". International Journal of Molecular Sciences 21 (3): 758. January 2020. doi:10.3390/ijms21030758. PMID 31979397.
- ↑ "Mutations affecting segment number and polarity in Drosophila". Nature 287 (5785): 795–801. October 1980. doi:10.1038/287795a0. PMID 6776413. Bibcode: 1980Natur.287..795N.
- ↑ "A functionally conserved homolog of the Drosophila segment polarity gene hh is expressed in tissues with polarizing activity in zebrafish embryos". Cell 75 (7): 1431–1444. December 1993. doi:10.1016/0092-8674(93)90628-4. PMID 8269519.
- ↑ "Sonic hedgehog, a member of a family of putative signaling molecules, is implicated in the regulation of CNS polarity". Cell 75 (7): 1417–1430. December 1993. doi:10.1016/0092-8674(93)90627-3. PMID 7916661.
- ↑ 9.0 9.1 "Sonic hedgehog mediates the polarizing activity of the ZPA". Cell 75 (7): 1401–1416. December 1993. doi:10.1016/0092-8674(93)90626-2. PMID 8269518.
- ↑ "Biologists Find Key Genes That Shape Patterning of Embryos". The New York Times. 1994-01-11. https://www.nytimes.com/1994/01/11/science/biologists-find-key-genes-that-shape-patterning-of-embryos.html?pagewanted=all&src=pm.
- ↑ Emus Can't Walk Backwards. Ebury Press. 2007-09-06. pp. 113–114. ISBN 978-0-09-192151-4. https://books.google.com/books?id=00U4ECEqJ0kC&q=sonic. Retrieved 2016-10-06.
- ↑ "Pokémon blocks gene name". Nature 438 (897): 897. 2005-12-15. doi:10.1038/438897a. PMID 16355177. Bibcode: 2005Natur.438..897S.
- ↑ 13.0 13.1 "A Gene Named Sonic". The New York Times. 1994-01-11. https://www.nytimes.com/1994/01/11/science/a-gene-named-sonic.html.
- ↑ "Cliff Tabin: Super Sonic An Interview". The Weekly Murmur. April 12, 2004. http://web.med.harvard.edu/sites/murmur/html/articles/041204/041204_akeen.asp.
- ↑ Riddle R. Ingenious: The Cyclops Gene. BBC.co.uk. Interviewed by Kat Arney. BBC Radio.
- ↑ "Zebrafish SHHa". University of Oregon. http://zfin.org/cgi-bin/webdriver?MIval=aa-markerview.apg&OID=ZDB-GENE-980526-166.
- ↑ "Zebrafish SHHb". University of Oregon. http://zfin.org/cgi-bin/webdriver?MIval=aa-markerview.apg&OID=ZDB-GENE-980526-41.
- ↑ "Induction of a specific muscle cell type by a hedgehog-like protein in zebrafish". Nature 382 (6590): 452–455. August 1996. doi:10.1038/382452a0. PMID 8684485. Bibcode: 1996Natur.382..452C.
- ↑ "Adenohypophysis formation in the zebrafish and its dependence on sonic hedgehog". Developmental Biology 254 (1): 36–49. February 2003. doi:10.1016/S0012-1606(02)00124-0. PMID 12606280.
- ↑ "Patterning the dorsal telencephalon: a role for sonic hedgehog?". The Journal of Neuroscience 27 (43): 11595–11603. October 2007. doi:10.1523/JNEUROSCI.3204-07.2007. PMID 17959802.
- ↑ "Hedgehog signaling is required for primary motoneuron induction in zebrafish". Development 128 (18): 3485–3495. September 2001. doi:10.1242/dev.128.18.3485. PMID 11566854.
- ↑ Principles of Development (5th ed.). Oxford University Press. 2015. pp. 500.
- ↑ "Sonic hedgehog regulates growth and morphogenesis of the tooth". Development 127 (22): 4775–4785. November 2000. doi:10.1242/dev.127.22.4775. PMID 11044393.
- ↑ "Hedgehog signalling from the zona limitans intrathalamica orchestrates patterning of the zebrafish diencephalon". Development 133 (5): 855–864. March 2006. doi:10.1242/dev.02248. PMID 16452095.
- ↑ "Shh and Gli3 regulate formation of the telencephalic-diencephalic junction and suppress an isthmus-like signaling source in the forebrain". Developmental Biology 359 (2): 242–250. November 2011. doi:10.1016/j.ydbio.2011.08.026. PMID 21925158.
- ↑ "Molecular subgroups of medulloblastoma: the current consensus". Acta Neuropathologica 123 (4): 465–472. April 2012. doi:10.1007/s00401-011-0922-z. PMID 22134537.
- ↑ "Pediatric medulloblastoma - update on molecular classification driving targeted therapies". Frontiers in Oncology 4: 176. 22 July 2014. doi:10.3389/fonc.2014.00176. PMID 25101241.
- ↑ "Paracrine sonic hedgehog signaling contributes significantly to acquired steroidogenesis in the prostate tumor microenvironment". International Journal of Cancer 140 (2): 358–369. January 2017. doi:10.1002/ijc.30450. PMID 27672740.
- ↑ "How limbs develop". Scientific American 280 (2): 74–79. February 1999. doi:10.1038/scientificamerican0299-74. PMID 9924814. Bibcode: 1999SciAm.280b..74R.
- ↑ "The morphogen sonic hedgehog is an axonal chemoattractant that collaborates with netrin-1 in midline axon guidance". Cell 113 (1): 11–23. April 2003. doi:10.1016/S0092-8674(03)00199-5. PMID 12679031.
- ↑ "Sonic hedgehog has a dual effect on the growth of retinal ganglion axons depending on its concentration". The Journal of Neuroscience 25 (13): 3432–3441. March 2005. doi:10.1523/JNEUROSCI.4938-04.2005. PMID 15800198.
- ↑ "Developmental basis for hind-limb loss in dolphins and origin of the cetacean bodyplan". Proceedings of the National Academy of Sciences of the United States of America 103 (22): 8414–8418. May 2006. doi:10.1073/pnas.0602920103. PMID 16717186. Bibcode: 2006PNAS..103.8414T.
- ↑ 33.0 33.1 33.2 "ENSG00000164690". Ensembl release 99. https://uswest.ensembl.org/Homo_sapiens/Gene/StructuralVariation_Gene?g=ENSG00000164690;r=7:155799980-155812463.
- ↑ 34.0 34.1 34.2 34.3 "UniprotKB - Q15465 (SHH_HUMAN)". UniProt Consortium. https://www.uniprot.org/uniprot/Q15465#.
- ↑ 35.0 35.1 35.2 35.3 35.4 "SHH gene". U.S. National Library of Medicine. https://ghr.nlm.nih.gov/gene/SHH#location.
- ↑ de Lussanet, M.H.E.; Osse, J.W.M. (2012). "An ancestral axial twist explains the contralateral forebain and the optic chiasm in vertebrates". Animal Biology 62 (2): 193–216. doi:10.1163/157075611X617102.
- ↑ "Control of Shh activity and signaling in the neural tube". Developmental Dynamics 219 (2): 143–154. October 2000. doi:10.1002/1097-0177(2000)9999:9999<::AID-DVDY1050>3.0.CO;2-Q. PMID 11002335.
- ↑ "The role of the notochord and floor plate in inductive interactions". Current Opinion in Genetics & Development 5 (4): 499–506. August 1995. doi:10.1016/0959-437X(95)90055-L. PMID 7580143.
- ↑ "The relationships between notochord and floor plate in vertebrate development revisited". Proceedings of the National Academy of Sciences of the United States of America 95 (20): 11733–11738. September 1998. doi:10.1073/pnas.95.20.11733. PMID 9751734. Bibcode: 1998PNAS...9511733T.
- ↑ "Induction of an additional floor plate in the neural tube". Acta Morphologica Neerlando-Scandinavica 23 (2): 91–97. October 1985. PMID 3834777.
- ↑ "The role of Sonic hedgehog in neural tube patterning". Cellular and Molecular Life Sciences 57 (12): 1695–1708. November 2000. doi:10.1007/PL00000652. PMID 11130176.
- ↑ "Requirement of 19K form of Sonic hedgehog for induction of distinct ventral cell types in CNS explants". Nature 375 (6529): 322–325. May 1995. doi:10.1038/375322a0. PMID 7753196. Bibcode: 1995Natur.375..322M.
- ↑ 43.0 43.1 43.2 "Two critical periods of Sonic Hedgehog signaling required for the specification of motor neuron identity". Cell 87 (4): 661–673. November 1996. doi:10.1016/S0092-8674(00)81386-0. PMID 8929535.
- ↑ "Cyclopia and defective axial patterning in mice lacking Sonic hedgehog gene function". Nature 383 (6599): 407–413. October 1996. doi:10.1038/383407a0. PMID 8837770. Bibcode: 1996Natur.383..407C.
- ↑ "Mesodermal control of neural cell identity: floor plate induction by the notochord". Science 250 (4983): 985–988. November 1990. doi:10.1126/science.2237443. PMID 2237443. Bibcode: 1990Sci...250..985P.
- ↑ 46.0 46.1 46.2 "The mechanisms of dorsoventral patterning in the vertebrate neural tube". Developmental Biology 282 (1): 1–13. June 2005. doi:10.1016/j.ydbio.2005.02.027. PMID 15936325.
- ↑ "The tumour-suppressor gene patched encodes a candidate receptor for Sonic hedgehog". Nature 384 (6605): 129–134. November 1996. doi:10.1038/384129a0. PMID 8906787. Bibcode: 1996Natur.384..129S.
- ↑ "Regulation of patched by sonic hedgehog in the developing neural tube". Proceedings of the National Academy of Sciences of the United States of America 93 (18): 9346–9351. September 1996. doi:10.1073/pnas.93.18.9346. PMID 8790332. Bibcode: 1996PNAS...93.9346M.
- ↑ 49.0 49.1 "Graded sonic hedgehog signaling and the specification of cell fate in the ventral neural tube". Cold Spring Harbor Symposia on Quantitative Biology 62: 451–466. 1997. doi:10.1101/SQB.1997.062.01.053. PMID 9598380.
- ↑ "Pax6 controls progenitor cell identity and neuronal fate in response to graded Shh signaling". Cell 90 (1): 169–180. July 1997. doi:10.1016/S0092-8674(00)80323-2. PMID 9230312.
- ↑ "The Hedgehog response network: sensors, switches, and routers". Science 304 (5678): 1755–1759. June 2004. doi:10.1126/science.1098020. PMID 15205520. Bibcode: 2004Sci...304.1755L.
- ↑ 52.0 52.1 "Combinatorial Gli gene function in floor plate and neuronal inductions by Sonic hedgehog". Development 125 (12): 2203–2212. June 1998. doi:10.1242/dev.125.12.2203. PMID 9584120.
- ↑ "Mouse Gli1 mutants are viable but have defects in SHH signaling in combination with a Gli2 mutation". Development 127 (8): 1593–1605. April 2000. doi:10.1242/dev.127.8.1593. PMID 10725236.
- ↑ "Gli2 is required for induction of floor plate and adjacent cells, but not most ventral neurons in the mouse central nervous system". Development 125 (15): 2759–2770. August 1998. doi:10.1242/dev.125.15.2759. PMID 9655799.
- ↑ 55.0 55.1 55.2 "Specification of ventral neuron types is mediated by an antagonistic interaction between Shh and Gli3". Nature Neuroscience 3 (10): 979–985. October 2000. doi:10.1038/79916. PMID 11017169.
- ↑ "Regulation of Gli2 and Gli3 activities by an amino-terminal repression domain: implication of Gli2 and Gli3 as primary mediators of Shh signaling". Development 126 (17): 3915–3924. September 1999. doi:10.1242/dev.126.17.3915. PMID 10433919.
- ↑ "Dorsal-ventral patterning of the spinal cord requires Gli3 transcriptional repressor activity". Genes & Development 16 (22): 2865–2878. November 2002. doi:10.1101/gad.243402. PMID 12435629.
- ↑ "Vertebrate Hedgehog signalling modulated by induction of a Hedgehog-binding protein". Nature 397 (6720): 617–621. February 1999. doi:10.1038/17611. PMID 10050855. Bibcode: 1999Natur.397..617C.
- ↑ "Sonic hedgehog synergizes with the extracellular matrix protein vitronectin to induce spinal motor neuron differentiation". Development 127 (2): 333–342. January 2000. doi:10.1242/dev.127.2.333. PMID 10603350.
- ↑ "A homeodomain protein code specifies progenitor cell identity and neuronal fate in the ventral neural tube". Cell 101 (4): 435–445. May 2000. doi:10.1016/S0092-8674(00)80853-3. PMID 10830170.
- ↑ "Regulation of the neural patterning activity of sonic hedgehog by secreted BMP inhibitors expressed by notochord and somites". Development 127 (22): 4855–4866. November 2000. doi:10.1242/dev.127.22.4855. PMID 11044400.
- ↑ "Noggin-mediated antagonism of BMP signaling is required for growth and patterning of the neural tube and somite". Genes & Development 12 (10): 1438–1452. May 1998. doi:10.1101/gad.12.10.1438. PMID 9585504.
- ↑ "Floor plate and motor neuron induction by different concentrations of the amino-terminal cleavage product of sonic hedgehog autoproteolysis". Cell 81 (3): 445–455. May 1995. doi:10.1016/0092-8674(95)90397-6. PMID 7736596.
- ↑ 64.0 64.1 64.2 64.3 64.4 64.5 64.6 64.7 64.8 64.9 "Establishing and interpreting graded Sonic Hedgehog signaling during vertebrate neural tube patterning: the role of negative feedback". Cold Spring Harbor Perspectives in Biology 1 (2): a002014. August 2009. doi:10.1101/cshperspect.a002014. PMID 20066087.
- ↑ "Wnt signaling plays an essential role in neuronal specification of the dorsal spinal cord". Genes & Development 16 (5): 548–553. March 2002. doi:10.1101/gad.937102. PMID 11877374.
- ↑ 66.0 66.1 "Notochord-derived Shh concentrates in close association with the apically positioned basal body in neural target cells and forms a dynamic gradient during neural patterning". Development 135 (6): 1097–1106. March 2008. doi:10.1242/dev.013086. PMID 18272593.
- ↑ "Boolean modelling reveals new regulatory connections between transcription factors orchestrating the development of the ventral spinal cord". PLOS ONE 9 (11): e111430. November 2014. doi:10.1371/journal.pone.0111430. PMID 25398016. Bibcode: 2014PLoSO...9k1430L.
- ↑ "miR-199 family contributes to regulation of sonic hedgehog expression during craniofacial development". Developmental Dynamics 249 (9): 1062–1076. September 2020. doi:10.1002/dvdy.191. PMID 32391617.
- ↑ Ten Cate's Oral Histology: Development, Structure, and Function (8th ed.). St. Louis, Mo.: Elsevier. 2012. ISBN 978-0-323-07846-7.
- ↑ "Epithelial-mesenchymal signalling regulating tooth morphogenesis". Journal of Cell Science 116 (Pt 9): 1647–1648. May 2003. doi:10.1242/jcs.00410. PMID 12665545.
- ↑ "The Shh signalling pathway in tooth development: defects in Gli2 and Gli3 mutants". Development 125 (15): 2803–2811. August 1998. doi:10.1242/dev.125.15.2803. PMID 9655803.
- ↑ Principles of Development (5th ed.). Oxford University Press. 2015. p. 500. ISBN 978-0-19-967814-3.
- ↑ "Involvement of Sonic hedgehog (Shh) in mouse embryonic lung growth and morphogenesis". Development 124 (1): 53–63. January 1997. doi:10.1242/dev.124.1.53. PMID 9006067.
- ↑ 74.0 74.1 74.2 74.3 74.4 "Sonic hedgehog regulates branching morphogenesis in the mammalian lung". Current Biology 8 (19): 1083–1086. September 1998. doi:10.1016/S0960-9822(98)70446-4. PMID 9768363.
- ↑ "FGF9 and SHH signaling coordinate lung growth and development through regulation of distinct mesenchymal domains". Development 133 (8): 1507–1517. April 2006. doi:10.1242/dev.02313. PMID 16540513.
- ↑ Modeling lung branching morphogenesis.. 81. 2008. 291–310. doi:10.1016/S0070-2153(07)81010-6. ISBN 9780123742537.
- ↑ 77.0 77.1 77.2 "Sonic hedgehog signaling in the lung. From development to disease". American Journal of Respiratory Cell and Molecular Biology 52 (1): 1–13. January 2015. doi:10.1165/rcmb.2014-0132TR. PMID 25068457.
- ↑ "Regulation of early lung morphogenesis: questions, facts and controversies". Development 133 (9): 1611–1624. May 2006. doi:10.1242/dev.02310. PMID 16613830.
- ↑ "Sonic hedgehog initiates cochlear hair cell regeneration through downregulation of retinoblastoma protein". Biochemical and Biophysical Research Communications 430 (2): 700–705. January 2013. doi:10.1016/j.bbrc.2012.11.088. PMID 23211596.
- ↑ "Hair follicles' transit-amplifying cells govern concurrent dermal adipocyte production through Sonic Hedgehog". Genes & Development 30 (20): 2325–2338. October 2016. doi:10.1101/gad.285429.116. PMID 27807033.
- ↑ "Proteolytic processing yields two secreted forms of sonic hedgehog". Molecular and Cellular Biology 15 (4): 2294–2303. April 1995. doi:10.1128/MCB.15.4.2294. PMID 7891723.
- ↑ "Mechanisms and functions of Hedgehog signalling across the metazoa". Nature Reviews. Genetics 12 (6): 393–406. June 2011. doi:10.1038/nrg2984. PMID 21502959.
- ↑ "Cholesterol modification of hedgehog signaling proteins in animal development". Science 274 (5285): 255–259. October 1996. doi:10.1126/science.274.5285.255. PMID 8824192. Bibcode: 1996Sci...274..255P.
- ↑ "Identification of a palmitic acid-modified form of human Sonic hedgehog". The Journal of Biological Chemistry 273 (22): 14037–14045. May 1998. doi:10.1074/jbc.273.22.14037. PMID 9593755.
- ↑ "A small molecule that binds Hedgehog and blocks its signaling in human cells". Nature Chemical Biology 5 (3): 154–156. March 2009. doi:10.1038/nchembio.142. PMID 19151731.
- ↑ "Humour of gene names lost in translation to patients". Nature 439 (7074): 266. January 2006. doi:10.1038/439266d. PMID 16421543. Bibcode: 2006Natur.439..266M.
- ↑ "Problems in the naming of genes". American Journal of Medical Genetics. Part A 140 (13): 1483–1484. July 2006. doi:10.1002/ajmg.a.31264. PMID 16718675.
- ↑ "Sonic Hedgehog, DICER, and the Problem With Naming Genes". Pacific Standard. September 26, 2014. https://psmag.com/environment/sonic-hedgehog-dicer-problem-naming-genes-91386.
- ↑ "Troublesome gene names get the boot". Nature: news061106–2. November 6, 2006. doi:10.1038/news061106-2. https://www.nature.com/news/2006/061106/full/news061106-2.html. Retrieved December 24, 2020.
Further reading
- "Sonic Hedgehog, a key development gene, experienced intensified molecular evolution in primates". Human Molecular Genetics 15 (13): 2031–2037. July 2006. doi:10.1093/hmg/ddl123. PMID 16687440.
- Developmental biology (6th ed.). Sunderland, Mass: Sinauer Associates. 2000. ISBN 978-0-87893-243-6. https://archive.org/details/developmentalbio00gilb.
- "The VACTERL association: lessons from the Sonic hedgehog pathway". Clinical Genetics 59 (5): 306–315. May 2001. doi:10.1034/j.1399-0004.2001.590503.x. PMID 11359461.
- "Shh signaling and pancreatic cancer: implications for therapy?". Cell Cycle 6 (13): 1553–1557. July 2007. doi:10.4161/cc.6.13.4467. PMID 17611415.
- "Pathways and consequences: Hedgehog signaling in human disease". Trends in Cell Biology 12 (12): 562–569. December 2002. doi:10.1016/S0962-8924(02)02405-4. PMID 12495844.
- "SHH mutation is associated with solitary median maxillary central incisor: a study of 13 patients and review of the literature". American Journal of Medical Genetics 102 (1): 1–10. July 2001. doi:10.1002/1096-8628(20010722)102:1<1::AID-AJMG1336>3.0.CO;2-U. PMID 11471164.
- "Hedgehog and spinal cord injury". Expert Opinion on Therapeutic Targets 9 (6): 1137–1145. December 2005. doi:10.1517/14728222.9.6.1137. PMID 16300466.
External links
- An introductory article on SHH at Davidson College
- Rediscovering biology: Unit 7 Genetics of development .. Expert interview transcripts interview with John Incardona PhD .. explanation of the discovery and naming of the sonic hedgehog gene
- ‘Sonic Hedgehog’ sounded funny at first .. New York Times November 12, 2006 ..
- GeneReviews/NCBI/NIH/UW entry on Anophthalmia / Microphthalmia Overview
- SHH – sonic hedgehog US National Library of Medicine
- Overview of all the structural information available in the PDB for UniProt: Q15465 (Human Sonic hedgehog protein) at the PDBe-KB.
- Overview of all the structural information available in the PDB for UniProt: Q62226 (Mouse Sonic hedgehog protein) at the PDBe-KB.
 |