Biology:XIST
 Generic protein structure example |
Xist (X-inactive specific transcript) is a non-coding RNA transcribed from the X chromosome of the placental mammals that acts as a major effector of the X-inactivation process.[1] It is a component of the Xic – X-chromosome inactivation centre[2] – along with two other RNA genes (Jpx and Ftx) and two protein genes (Tsx and Cnbp2).[3]
The Xist RNA, a large (17 kb in humans)[4] transcript, is expressed on the inactive chromosome and not on the active one. It is processed in a similar way to mRNAs, through splicing and polyadenylation. However, it remains untranslated. It has been suggested that this RNA gene evolved at least partly from a protein-coding gene that became a pseudogene.[5] The inactive X chromosome is coated with this transcript, which is essential for the inactivation.[6] X chromosomes lacking Xist will not be inactivated, while duplication of the Xist gene on another chromosome causes inactivation of that chromosome.[7]
The human Xist gene was discovered by Andrea Ballabio through a cDNA library screening and then characterized in collaboration with Carolyn J. Brown and Hunt Willard.[8][9]
Function
X-inactivation is an early developmental process in mammalian females that transcriptionally silences one of the pair of X chromosomes, thus providing dosage equivalence between males and females (see dosage compensation). The process is regulated by several factors, including a region of chromosome X called the X-inactivation center (XIC). The XIST gene is expressed exclusively from the XIC of the inactive X chromosome. The transcript is spliced but apparently does not encode a protein. The transcript remains in the nucleus where it coats the inactive X chromosome. Alternatively spliced transcript variants have been identified, but their full length sequences have not been determined.[1]
The functional role of the Xist transcript was definitively demonstrated in mouse female ES cells using a novel antisense technology, called peptide nucleic acid (PNA) interference mapping. In the reported experiments, a single 19-bp antisense cell-permeating PNA targeted against a particular region of Xist RNA prevented the formation of Xi and inhibited cis-silencing of X-linked genes. The association of the Xi with macro-histone H2A is also disturbed by PNA interference mapping.[10] The X-inactivation process occurs in mice even in the absence of this gene via epigenetic regulation, but Xist is required to stabilize this silencing.[11]
In addition to being expressed in nearly all females, XIST is expressed in narrow developmental contexts in males including human preimplantation embryos, primordial germ cells, testicular germ cell tumors, and a subset of male cancers of diverse lineages.[12] It may be involved in the dosage compensation of supernumerary X chromosomes in the latter two cases.
Gene location
The human Xist RNA gene is located on the long (q) arm of the X chromosome. The Xist RNA gene consists of conserved repeats within its structure and is also largely localized in the nucleus.[4] The Xist RNA gene consists of an A region, which contains 8 repeats separated by U-rich spacers. The A region appears to contain two long stem-loop structures that each include four repeats.[13] An ortholog of the Xist RNA gene in humans has been identified in mice.[14][15] This ortholog is a 15 kb Xist RNA gene that is also localized in the nucleus. However, the ortholog does not consist of conserved repeats.[16] The gene also consists of an Xist Inactivation Center (XIC), which plays a major role in X-inactivation.[17]
Transcript organization
A region
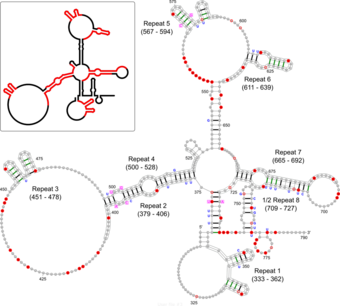
The Xist RNA contains a region of conservation called the repeat A (repA) region that contains up to nine repeated elements.[13] It was initially suggested that repA repeats could fold back on themselves to form local intra-repeat stem-loop structures. Later work using in vitro biochemical structure probing proposed several inter-repeat stem-loop structures.[4][13] A recent study using in vivo biochemical probing and comparative sequence analysis proposed a revision of the repA structure model that includes both intra-repeat and inter-repeat folding found in previous models as well as novel features (see Figure). In addition to its agreement with the in vivo data, this revised model is highly conserved in rodents and mammals (including humans) suggesting functional importance for repA structure. Although the exact function of the repA region is uncertain, it was shown that the entire region is needed for efficient binding to the Suz12 protein.[13]
C region
The Xist RNA directly binds to the inactive X-chromosome through a chromatin binding region of the RNA transcript. The Xist chromatin binding region was first elucidated in female mouse fibroblastic cells. The primary chromatin binding region was shown to localize to the C-repeat region. The chromatin-binding region was functionally mapped and evaluated by using an approach for studying noncoding RNA function in living cells called peptide nucleic acid (PNA) interference mapping. In the reported experiments, a single 19-bp antisense cell-permeating PNA targeted against a particular region of Xist RNA caused the disruption of the Xi. The association of the Xi with macro-histone H2A is also disturbed by PNA interference mapping.[10]
X-inactivation centre (XIC)
The Xist RNA gene lies within the X-inactivation centre (XIC), which plays a major role in Xist expression and X-inactivation.[18] The XIC is located on the q arm of the X chromosome (Xq13). XIC regulates Xist in cis X-inactivation, where Tsix, an antisense of Xist, downregulates the expression of Xist. The Xist promoter of XIC is the master regulator of X-inactivation.[17] X-inactivation plays a key role in dosage compensation.
Tsix antisense transcript
The Tsix antisense gene is a transcript of the Xist gene at the XIC center.[19] The Tsix antisense transcript acts in cis to repress the transcription of Xist, which negatively regulates its expression. The mechanism behind how Tsix modulates Xist activity in cis is poorly understood; however, there are a few theories on its mechanism. One theory is that Tsix is involved in chromatin modification at the Xist locus and another is that transcription factors of pluripotent cells play a role in Xist repression.[20]
Regulation of the Xist promoter
Methylation
The Tsix antisense is believed to activate DNA methyl transferases that methylate the Xist promoter, in return resulting in inhibition of the Xist promoter and thus the expression of the Xist gene.[21] Methylation of histone 3 lysine 4 (H3K4) produces an active chromatin structure, which recruits transcription factors and thus allows for transcription to occur, therefore in this case the transcription of Xist.[22]
dsRNA and RNAi
A dsRNA and RNAi pathway have been also proposed to play a role in regulation of the Xist Promoter. Dicer is an RNAi enzyme and it is believed to cleave the duplex of Xist and Tsix at the beginning of X-inactivation, to small ~30 nucleotide RNAs, which have been termed xiRNAs, These xiRNAs are believed to be involved in repressing Xist on the probable active X chromosome based upon studies. A study was conducted where normal endogenous Dicer levels were decreased to 5%, which led to an increase in Xist expression in undifferentiated cells, thus supporting the role of xiRNAs in Xist repression.[23] The role and mechanism of xiRNAs is still under examination and debate.[citation needed]
Tsix independent mechanisms
Pluripotent cell transcriptional factors
Pluripotent stem cells express transcription factors Nanog, Oct4 and Sox2 that seem to play a role in repressing Xist. In the absence of Tsix in pluripotent cells, Xist is repressed, where a mechanism has been proposed that these transcription factors cause splicing to occur at intron 1 at the binding site of these factors on the Xist gene, which inhibits Xist expression[20] A study was conducted where Nanog or Oct4 transcription factors were depleted in pluripotent cells, which resulted in the upregulation of Xist. From this study, it is proposed that Nanog and Oct4 are involved in the repression of Xist expression.[24]
Polycomb repressive complex
Polycomb repressive complex 2 (PRC2) consist of a class of polycomb group proteins that are involved in catalyzing the trimethylation of histone H3 on lysine 27 (K27), which results in chromatin repression, and thus leads to transcriptional silencing. Xist RNA recruits polycomb complexes to the inactive X chromosome at the onset of XCI.[25] SUZ12 is a component of the PRC2 and contains a zinc finger domain. The zinc finger domain is believed to bind to the RNA molecule.[26] The PRC2 has been observed to repress Xist expression independent of the Tsix antisense transcript, although the definite mechanism is still not known.
Dosage compensation
X-inactivation plays a key role in dosage compensation mechanisms that allow for equal expression of the X and autosomal chromosomes.[27] Different species have different dosage compensation methods, with all of the methods involving the regulation of an X chromosome from one of the either sexes.[27] Some methods involved in dosage compensation to inactivate one of the X chromosomes from one of the sexes are Tsix antisense gene, DNA methylation and DNA acetylation;[28] however, the definite mechanism of X-inactivation is still poorly understood. If one of the X chromosomes is not inactivated or is partially expressed, it could lead to over expression of the X chromosome and it could be lethal in some cases.
Turner's Syndrome is one example of where dosage compensation does not equally express the X chromosome, and in females one of the X chromosomes is missing or has abnormalities, which leads to physical abnormalities and also gonadal dysfunction in females due to the one missing or abnormal X chromosome. Turner's syndrome is also referred to as a monosomy X condition.[29]
X-inactivation cycle
Xist expression and X-inactivation change throughout embryonic development. In early embryogenesis, the oocyte and sperm do not express Xist and the X chromosome remains active. After fertilization, when the cells are in the 2 to 4 cell stage, Xist transcripts are expressed from paternal X chromosome(Xp) in every cell, causing that X chromosome to become imprinted and inactivated. Some cells develop into pluripotent cells (the inner cell mass) when the blastocyte forms. There, the imprint is removed, leading to the downregulation of Xist and thus reactivation of the inactive X chromosome. Recent data suggests that Xist activity is regulated by an anti-sense transcript.[30] The epiblast cells are then formed and they begin to be differentiated, and the Xist is upregulated from either of the two X chromosomes and at random in ICM, but the Xist is maintained in epiblast, an X is inactivated and the Xist allele is turned off in the active X chromosome. In maturing XX primordial germ cells, Xist is downregulated and X reactivation occurs once again.[31]
Disease linkage
Mutations in the XIST promoter cause familial skewed X-inactivation.[1]
Interactions
XIST has been shown to interact with BRCA1.[32][33]
See also
References
- ↑ 1.0 1.1 1.2 "Entrez Gene: XIST X (inactive)-specific transcript". https://www.ncbi.nlm.nih.gov/sites/entrez?Db=gene&Cmd=ShowDetailView&TermToSearch=7503.
- ↑ "Silencing of the mammalian X chromosome". Annual Review of Genomics and Human Genetics 6: 69–92. 2005. doi:10.1146/annurev.genom.6.080604.162350. PMID 16124854.
- ↑ "Comparative sequence analysis of the X-inactivation center region in mouse, human, and bovine". Genome Research 12 (6): 894–908. June 2002. doi:10.1101/gr.152902. PMID 12045143.
- ↑ 4.0 4.1 4.2 "The human XIST gene: analysis of a 17 kb inactive X-specific RNA that contains conserved repeats and is highly localized within the nucleus". Cell 71 (3): 527–42. October 1992. doi:10.1016/0092-8674(92)90520-M. PMID 1423611.
- ↑ "The Xist RNA gene evolved in eutherians by pseudogenization of a protein-coding gene". Science 312 (5780): 1653–1655. June 2006. doi:10.1126/science.1126316. PMID 16778056. Bibcode: 2006Sci...312.1653D.
- ↑ "Xist and the order of silencing" (Review Article). EMBO Reports 8 (1): 34–39. January 2007. doi:10.1038/sj.embor.7400871. PMID 17203100. "Figure 1 Xist RNA encompasses the X from which it is transcribed.".
- ↑ "Requirement for Xist in X chromosome inactivation". Nature 379 (6561): 131–7. 1996. doi:10.1038/379131a0. PMID 8538762. Bibcode: 1996Natur.379..131P.

- ↑ "A gene from the region of the human X inactivation centre is expressed exclusively from the inactive X chromosome". Nature 349 (6304): 38–44. Jan 1991. doi:10.1038/349038a0. PMID 1985261. Bibcode: 1991Natur.349...38B.
- ↑ "Gracefully ageing at 50, X-chromosome inactivation becomes a paradigm for RNA and chromatin control". Nature Reviews Molecular Cell Biology 12 (12): 815–26. 2011. doi:10.1038/nrm3231. PMID 22108600.
- ↑ 10.0 10.1 "PNA interference mapping demonstrates functional domains in the noncoding RNA Xist". Proceedings of the National Academy of Sciences of the United States of America 98 (16): 9215–20. July 2001. doi:10.1073/pnas.161173098. PMID 11481485. Bibcode: 2001PNAS...98.9215B.
- ↑ "Evidence of Xist RNA-independent initiation of mouse imprinted X-chromosome inactivation". Nature 460 (7255): 647–51. July 2009. doi:10.1038/nature08161. PMID 19571810. Bibcode: 2009Natur.460..647K.
- ↑ Sadagopan, Ananthan; Nasim, Imran T.; Li, Jiao; Achom, Mingkee; Zhang, Cheng-Zhong; Viswanathan, Srinivas R. (2022-11-16). "Somatic XIST activation and features of X chromosome inactivation in male human cancers" (in English). Cell Systems 13 (11): 932–944.e5. doi:10.1016/j.cels.2022.10.002. ISSN 2405-4712. PMID 36356577.
- ↑ 13.0 13.1 13.2 13.3 "2-D structure of the A region of Xist RNA and its implication for PRC2 association". PLOS Biology 8 (1): e1000276. January 2010. doi:10.1371/journal.pbio.1000276. PMID 20052282.
- ↑ "Characterization of a murine gene expressed from the inactive X chromosome". Nature 351 (6324): 325–9. May 1991. doi:10.1038/351325a0. PMID 2034278. Bibcode: 1991Natur.351..325B.
- ↑ "Conservation of position and exclusive expression of mouse Xist from the inactive X chromosome". Nature 351 (6324): 329–31. May 1991. doi:10.1038/351329a0. PMID 2034279. Bibcode: 1991Natur.351..329B.
- ↑ "The product of the mouse Xist gene is a 15 kb inactive X-specific transcript containing no conserved ORF and located in the nucleus". Cell 71 (3): 515–26. 1992. doi:10.1016/0092-8674(92)90519-I. PMID 1423610.
- ↑ 17.0 17.1 "Tsix, a gene antisense to Xist at the X-inactivation centre". Nature Genetics 21 (4): 400–4. April 1999. doi:10.1038/7734. PMID 10192391.
- ↑ "Xist has properties of the X-chromosome inactivation centre". Nature 386 (6622): 272–5. March 1997. doi:10.1038/386272a0. PMID 9069284. Bibcode: 1997Natur.386..272H.
- ↑ "Tsix, a gene antisense to Xist at the X-inactivation centre". Nature Genetics 21 (4): 400–4. April 1999. doi:10.1038/7734. PMID 10192391.
- ↑ 20.0 20.1 "Xist gene regulation at the onset of X inactivation". Current Opinion in Genetics & Development 19 (2): 122–6. April 2009. doi:10.1016/j.gde.2009.03.003. PMID 19345091.
- ↑ "Dicer regulates Xist promoter methylation in ES cells indirectly through transcriptional control of Dnmt3a". Epigenetics & Chromatin 1 (1): 2. October 2008. doi:10.1186/1756-8935-1-2. PMID 19014663.
- ↑ "Tsix transcription across the Xist gene alters chromatin conformation without affecting Xist transcription: implications for X-chromosome inactivation". Genes & Development 19 (12): 1474–84. June 2005. doi:10.1101/gad.341105. PMID 15964997.
- ↑ "Intersection of the RNA interference and X-inactivation pathways". Science 320 (5881): 1336–41. June 2008. doi:10.1126/science.1157676. PMID 18535243. Bibcode: 2008Sci...320.1336O.
- ↑ "Molecular coupling of Xist regulation and pluripotency". Science 321 (5896): 1693–5. September 2008. doi:10.1126/science.1160952. PMID 18802003. Bibcode: 2008Sci...321.1693N.
- ↑ "Polycomb proteins targeted by a short repeat RNA to the mouse X chromosome". Science 322 (5902): 750–6. October 2008. doi:10.1126/science.1163045. PMID 18974356. Bibcode: 2008Sci...322..750Z.
- ↑ "Polycomb group proteins Ring1A/B link ubiquitylation of histone H2A to heritable gene silencing and X inactivation". Developmental Cell 7 (5): 663–76. 2004. doi:10.1016/j.devcel.2004.10.005. PMID 15525528.
- ↑ 27.0 27.1 "Dosage compensation of the active X chromosome in mammals". Nature Genetics 38 (1): 47–53. January 2006. doi:10.1038/ng1705. PMID 16341221.
- ↑ "Synergism of Xist RNA, DNA methylation, and histone hypoacetylation in maintaining X chromosome inactivation". The Journal of Cell Biology 153 (4): 773–84. May 2001. doi:10.1083/jcb.153.4.773. PMID 11352938.
- ↑ "Dosage compensation of the X chromosome and Turner syndrome=International-Congress-series". International Congress Series 1298: 3–8. 2006. doi:10.1016/j.ics.2006.06.012.
- ↑ "Reactivation of the paternal X chromosome in early mouse embryos". Science 303 (5658): 666–9. January 2004. doi:10.1126/science.1092674. PMID 14752160. Bibcode: 2004Sci...303..666M.
- ↑ "Xist expression and macroH2A1.2 localisation in mouse primordial and pluripotent embryonic germ cells". Differentiation; Research in Biological Diversity 69 (4–5): 216–25. January 2002. doi:10.1046/j.1432-0436.2002.690415.x. PMID 11841480.
- ↑ "Association of BRCA1 with the inactive X chromosome and XIST RNA". Philosophical Transactions of the Royal Society of London. Series B, Biological Sciences 359 (1441): 123–8. January 2004. doi:10.1098/rstb.2003.1371. PMID 15065664.
- ↑ "BRCA1 supports XIST RNA concentration on the inactive X chromosome". Cell 111 (3): 393–405. November 2002. doi:10.1016/S0092-8674(02)01052-8. PMID 12419249.
Further reading
- "A gene from the region of the human X inactivation centre is expressed exclusively from the inactive X chromosome". Nature 349 (6304): 38–44. January 1991. doi:10.1038/349038a0. PMID 1985261. Bibcode: 1991Natur.349...38B.
- "Localization of the X inactivation centre on the human X chromosome in Xq13". Nature 349 (6304): 82–4. January 1991. doi:10.1038/349082a0. PMID 1985270. Bibcode: 1991Natur.349...82B.
- "XIST RNA paints the inactive X chromosome at interphase: evidence for a novel RNA involved in nuclear/chromosome structure". The Journal of Cell Biology 132 (3): 259–75. February 1996. doi:10.1083/jcb.132.3.259. PMID 8636206.
- "Identification and characterization of the human XIST gene promoter: implications for models of X chromosome inactivation". Nucleic Acids Research 25 (13): 2661–71. July 1997. doi:10.1093/nar/25.13.2661. PMID 9185579.
- "A promoter mutation in the XIST gene in two unrelated families with skewed X-chromosome inactivation". Nature Genetics 17 (3): 353–6. November 1997. doi:10.1038/ng1197-353. PMID 9354806.
- "A revision of the human XIST gene organization and structural comparison with mouse Xist". Mammalian Genome 11 (3): 220–4. March 2000. doi:10.1007/s003350010040. PMID 10723727.
- "An ectopic human XIST gene can induce chromosome inactivation in postdifferentiation human HT-1080 cells". Proceedings of the National Academy of Sciences of the United States of America 99 (13): 8677–82. June 2002. doi:10.1073/pnas.132468999. PMID 12072569. Bibcode: 2002PNAS...99.8677H.
- "BRCA1 supports XIST RNA concentration on the inactive X chromosome". Cell 111 (3): 393–405. November 2002. doi:10.1016/S0092-8674(02)01052-8. PMID 12419249.
- "The roles of supernumerical X chromosomes and XIST expression in testicular germ cell tumors". The Journal of Urology 169 (4): 1546–52. April 2003. doi:10.1097/01.ju.0000044927.23323.5a. PMID 12629412.
- "Familial cases of point mutations in the XIST promoter reveal a correlation between CTCF binding and pre-emptive choices of X chromosome inactivation". Human Molecular Genetics 14 (7): 953–65. April 2005. doi:10.1093/hmg/ddi089. PMID 15731119.
- "XIST repression in the absence of DNMT1 and DNMT3B". DNA Research 12 (5): 373–8. 2007. doi:10.1093/dnares/dsi013. PMID 16769694.
- "XIST RNA exhibits nuclear retention and exhibits reduced association with the export factor TAP/NXF1". Chromosoma 116 (4): 373–83. August 2007. doi:10.1007/s00412-007-0100-1. PMID 17333237.
- "Inducible XIST-dependent X-chromosome inactivation in human somatic cells is reversible". Proceedings of the National Academy of Sciences of the United States of America 104 (24): 10104–9. June 2007. doi:10.1073/pnas.0610946104. PMID 17537922. Bibcode: 2007PNAS..10410104C.
- "X inactive-specific transcript RNA coating and genetic instability of the X chromosome in BRCA1 breast tumors". Cancer Research 67 (11): 5134–40. June 2007. doi:10.1158/0008-5472.CAN-07-0465. PMID 17545591.
- "Xist RNA and the mechanism of X chromosome inactivation". Annual Review of Genetics 36: 233–78. 2002. doi:10.1146/annurev.genet.36.042902.092433. PMID 12429693.
- "X-chromosome inactivation: closing in on proteins that bind Xist RNA". Trends in Genetics 18 (7): 352–8. July 2002. doi:10.1016/S0168-9525(02)02717-8. PMID 12127775.
- "X chromosome inactivation is mediated by Xist RNA stabilization". Cell 90 (5): 907–16. September 1997. doi:10.1016/S0092-8674(00)80355-4. PMID 9298902.
- "A novel role for Xist RNA in the formation of a repressive nuclear compartment into which genes are recruited when silenced". Genes & Development 20 (16): 2223–37. August 2006. doi:10.1101/gad.380906. PMID 16912274.
- "Conservation of position and exclusive expression of mouse Xist from the inactive X chromosome". Nature 351 (6324): 329–31. May 1991. doi:10.1038/351329a0. PMID 2034279. Bibcode: 1991Natur.351..329B.
- "Advances in understanding chromosome silencing by the long non-coding RNA Xist". Philosophical Transactions of the Royal Society of London. Series B, Biological Sciences 368 (1609): 20110325. January 2013. doi:10.1098/rstb.2011.0325. PMID 23166390.
- "Long noncoding RNAs may alter chromosome's 3D structure". Science 340 (6135): 910. May 2013. doi:10.1126/science.340.6135.910-a. PMID 23704542. Bibcode: 2013Sci...340Q.910P.
External links
 |
