Chemistry:δ-Cadinol
| |
| Names | |
|---|---|
| IUPAC name
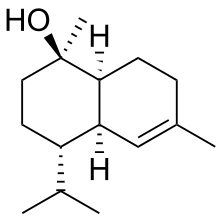
(1S,4S)-1,6-Dimethyl-4-propan-2-yl-3,4,4a,7,8,8a-hexahydro-2H-naphthalen-1-ol
| |
| Other names
Torreyol
1-epi-α-Cadinol 1β-Cadin-4-en-10-ol | |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChemSpider | |
PubChem CID
|
|
| |
| |
| Properties | |
| C15H26O | |
| Molar mass | 222.37 g/mol |
| Appearance | White crystalline needles |
| Melting point | 138 to 139 °C (280 to 282 °F; 411 to 412 K) |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
δ-Cadinol is an organic compound, a sesquiterpenoid alcohol produced by many plants as well as some animals and microorganisms. It is a white crystalline solid, soluble in isopropyl ether and ethanol. It is an epimer of α-cadinol.
δ-Cadinol exists in nature as either of two enantiomers distinguished by the prefixes (+)- and (−)-.[1][2] The (+)-isomer was identified by E. Shinozaki in 1922 from the leaves of Torreya nucifera and originally named torreyol.[1] The (−)-isomer was isolated in 1951 by Haagen-Smit and others from Pinus albicaulus and first called albicaulol.[1] Its structure was determined in 1970 by Lars Westfelt.[2] Other names were given to δ-cadinol based on its various biological sources before the structures were confirmed, including sesquigoyol for (+)-δ-cadinol and pilgerol for (−)-δ-cadinol.[2][3] Lambertol is thought to be either (+)-δ-cadinol or (−)-δ-cadinol.[2] Cedrelanol was originally thought to be identical to (−)-δ-cadinol but was later confirmed to have the structure of τ-cadinol.[4]
Occurrence
δ-Cadinol is produced by the fungus Xylobolus frustulatus as long white needles when grown in malt agar medium.[5] It also occurs in many conifers,[1] and in many other organisms including
- Achillea millefolium (6%)[6]
- Cedrela odorata[1]
- Clitocybe illudens (a mushroom)[3]
- Copaifera multijuga (1%; a major contributor to the aroma of copaiba oil)[7]
- Dictyopteris divaricata (a brown alga)[1]
- Plebejus argyrognomon (a butterfly; acts as a pheromone)[8]
See also
- α-Cadinol
- τ-Cadinol
References
- ↑ 1.0 1.1 1.2 1.3 1.4 1.5 Westfelt, Lars; Tränkner, Hans; Brandänge, Svante; Walle, Thomas; Sjöberg, Berndt; Bunnenberg, E.; Djerassi, Carl; Records, Ruth (1966). "(---)-Torreyol ("delta-Cadinol")". Acta Chemica Scandinavica 20: 2893–2894. doi:10.3891/acta.chem.scand.20-2893.
- ↑ 2.0 2.1 2.2 2.3 Lars Westfelt (1970), "(−)-Torryeol ('δ-Cadinol')". Acta Chemica Scandinavica volume 24 issue 5 16181622 doi:10.3891/acta.chem.scand.24-1618
- ↑ 3.0 3.1 Borg-Karlson, A; Norin, Torbjörn; Talvitie, Antti (1981). "Configurations and conformations of torreyol (δ-cadinol), α-cadinol, T-muurolol and T-cadinol". Tetrahedron 37 (22): 425. doi:10.1016/S0040-4020(01)92031-9.
- ↑ Smolders, R.R. (1967). "Structure et configuration absolue du cédrélanol ((−)-δ-cadinol), alcool sesquiterpénique C15H26O l'huile essentielle Cedrela odorata brasiliensis". Canadian Journal of Chemistry 45 (9): 889-896.
- ↑ Vaneijk, G; Roeijmans, H; Verwiel, P (1984). "Isolation and identification of the sesquiterpenoid (+)-torreyol fromXylobolus frustulatus". Experimental Mycology 8 (3): 273. doi:10.1016/0147-5975(84)90012-4.
- ↑ Kotan, Recep; Cakir, Ahmet; Dadasoglu, Fatih; Aydin, Tuba; Cakmakci, Ramazan; Ozer, Hakan; Kordali, Saban; Mete, Ebru et al. (2010). "Antibacterial activities of essential oils and extracts of TurkishAchillea, SaturejaandThymusspecies against plant pathogenic bacteria". Journal of the Science of Food and Agriculture 90 (1): 145–60. doi:10.1002/jsfa.3799. PMID 20355025.
- ↑ Sant'Anna, Beatriz M. P.; Fontes, Silvia Paredes; Pinto, Angelo C.; Rezende, Claudia M. (2007). "Characterization of woody odorant contributors in copaiba oil (Copaifera multijuga Hayne)". Journal of the Brazilian Chemical Society 18 (5): 984. doi:10.1590/S0103-50532007000500016.
- ↑ Lundgren, Lennart; Bergström, Gunnar (1976). "Wing scents and scent-released phases in the courtship behavior of Lycaeides argyrognomon (Lepidoptera: Lycaenidae)". Journal of Chemical Ecology 1 (4): 399. doi:10.1007/BF00988581.
 |

