Chemistry:1,1,1,2-Tetrachloropropane
From HandWiki
| |||
|
| |||
| Names | |||
|---|---|---|---|
| Preferred IUPAC name
1,1,1,2-Tetrachloropropane | |||
| Identifiers | |||
3D model (JSmol)
|
|||
| ChemSpider | |||
| EC Number |
| ||
PubChem CID
|
|||
CompTox Dashboard (EPA)
|
|||
| |||
| |||
| Properties | |||
| C3H4Cl4 | |||
| Molar mass | 181.87 g·mol−1 | ||
| Appearance | Colorless liquid | ||
| Melting point | −64 °C (−83 °F; 209 K) | ||
| Boiling point | 152.4 °C (306.3 °F; 425.5 K) | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |||
| Infobox references | |||
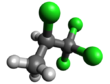
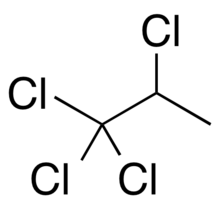
1,1,1,2-Tetrachloropropane is a compound of chlorine, hydrogen, and carbon. It has chemical formula C3H4Cl4. The structure has a propane skeleton, but four of the hydrogen atoms are replaced by chlorine atoms.
Preparation
1,1,1,2-Tetrachloropropane can be produced by addition of hydrogen chloride to 1,1,1-trichloropropene.[1]
- CCl3CH=CH2 + HCl → CCl3CHClCH3
References
- ↑ Price, Charles C.; Marshall, Howard D. (1943). "The Reaction of Anisole with 1,1,1-Trichloro-2-Methyl-2-Propene". The Journal of Organic Chemistry 08 (6): 532–535. doi:10.1021/jo01194a006. ISSN 0022-3263.
 |