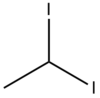
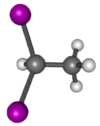
Chemistry:1,1-Diiodoethane
| |||
| Names | |||
|---|---|---|---|
| Preferred IUPAC name
1,1-Diiodoethane[1] | |||
| Other names
Ethylidene iodide
| |||
| Identifiers | |||
3D model (JSmol)
|
|||
| ChemSpider | |||
| EC Number |
| ||
PubChem CID
|
|||
| |||
| Properties | |||
| C2H4I2 | |||
| Molar mass | 281.863 g·mol−1 | ||
| Density | 3.0±0.1 g/cm3[2] | ||
| Boiling point | 154.7±23.0 °C | ||
| Solubility | most organic solvents | ||
| Hazards | |||
| GHS pictograms |  
| ||
| GHS Signal word | WARNING | ||
| P261, P264, P270, P271, P280, P301+312, P302+352, P304+340, P305+351+338, P312, P321, P330, P332+313, P337+313, P362, P403+233, P405, P501 | |||
| Flash point | 63.7±18.1 °C | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |||
| Infobox references | |||
1,1-Diiodoethane is an organic saturated haloalkane containing iodine with formula CH3CHI2.
Preparation
1,1-Diiodoethane can be synthesized from gem-dihaloalkanes. The starting material is 1,1-dichloroethane, and iodoethane is a source of iodine. In the presence of aluminium trichloride, 1,1-dichloroethane will converted to 1,1-diiodoethane.[3]
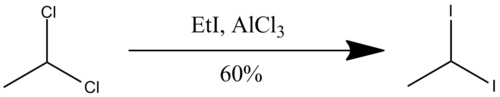
To be specific, mix 0.4 mol (~39.6 g) of 1,1-dichloroethane with 1.2 mol (~187 g) of ethyl iodide, and ~2.0 g of aluminium chloride. Heat for three hours using steam bath. Then, wash the mixture with H2O and NaHSO3 respectively, and dry with MgSO4. By boiling at 76-76 °C and 25 mmHg, about 67.3 g of product will be received when distilled.[4]
The alternative method, which does not require 1,1-dichloroethane, is the reaction of iodine, triethylamine and hydrazone of acetaldehyde. Using 1 mol of acetaldehyde, about 95 g, which is 34% from acetaldehyde, of 1,1-diiodoethane formed.[4]
Application
1,1-Diiodoethane is commonly used as a reactant in reaction such as SN2. The following are some examples of SN2 reaction using 1,1-diiodoethane as a reactant.[5]
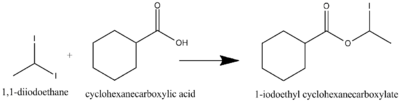
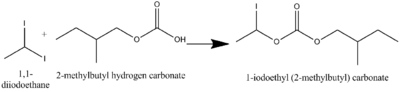
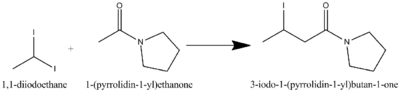
Moreover, is can also be used as a reactant in enolate substitution reaction as the following examples.[5]

See also
References
- ↑ "1,1-Diiodoethane - Compound Summary". PubChem Compound Database. USA: National Center for Biotechnology Information. Identification. https://pubchem.ncbi.nlm.nih.gov/compound/68980. Retrieved 7 June 2017.
- ↑ "CSID:8014297". ChemSpider. http://www.chemspider.com/Chemical-Structure.8014297.html. Retrieved 7 June 2017.
- ↑ 3.0 3.1 Benneche, T.; Challenger, S.; Chemla, F.; Cordier, C.; Demchenko, A. V.; De Meo, C.; Diaper, C. M.; Fascione, M. A. et al. (2007). Science of Synthesis: Houben-Weyl Methods of Molecular Transformations. 29. Thieme New York. pp. 106. ISBN 978-1-58890-461-4. https://books.google.com/books?id=VSGGAwAAQBAJ&q=1%2C1-diiodoethane&pg=PA106. Retrieved 8 June 2017.
- ↑ 4.0 4.1 Taschner, Michael J. (2001). "1,1-Diiodoethane". e-EROS Encyclopedia of Reagents for Organic Synthesis. John Wiley & Sons. doi:10.1002/047084289X.rd239. ISBN 978-0471936237.
- ↑ 5.0 5.1 5.2 5.3 5.4 5.5 "1,1-diiodoethane", Chemsink. Retrieved on 8 June 2017.
External links
- PubChem Compound Summary for CID 68980
- Chemistry of Ethylidene Moieties on Platinum Surfaces: 1,1-Diiodoethane on Pt(111)
 |