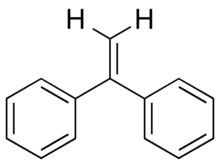
Chemistry:1,1-Diphenylethylene
From HandWiki
| |
| Names | |
|---|---|
| Preferred IUPAC name
1,1′-(Ethene-1,1-diyl)dibenzene | |
| Other names
Ethene-1,1-diyldibenzene
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| C14H12 | |
| Molar mass | 180.250 g·mol−1 |
| Melting point | 8 °C[1] |
| Boiling point | 277 °C[2] |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
1,1-Diphenylethylene is an aromatic hydrocarbon with chemical formula C14H12.
Properties
1,1-Diphenylethylene mediates the radical polymerization of methyl acrylate or styrene. Meditation by 1,1-Diphenylethylene generates low molecular weight polymer by a termination reaction.[3] Dibenzofulvene is an analogue of a 1,1-Diphenylethylene.[4]
Synthesis
1,1-Diphenylethylene is technical prepared by alkylating benzene by styrene in presence of a zeolite beta and subsequent dehydrogenation.[5]
- styrene + benzene → 1,1-diphenylethane → 1,1-diphenylethylene + H2
See also
- Dibenzofulvene
- Stilbene
References
- ↑ Smith, R.H.; Andrews, D.H.: Thermal Energy Studies I. Phenyl Derivatives of Methane, Ethane and Some Related Compounds in J. Am. Chem. Soc. 53 (1931) 3644.
- ↑ CRC Handbook of Data on Organic Compounds, 2nd Edition, Weast,R.C and Grasselli, J.G., ed(s)., CRC Press, Inc., Boca Raton, FL, 1989, 1.
- ↑ Minjian Zhao; Zhifeng Fu; Yan Shi; Wantai Yang (2015). "Polymerization Mechanism in the Presence of 1,1-Diphenylethylene Part 2: Synthesis and Characterization of PMA and PSt". Macromolecular Chemistry and Physics 216 (22): 2202–2210. doi:10.1002/macp.201500249.
- ↑ Tamaki Nakano; Kazuyuki Takewaki; Tohru Yade; Yoshio Okamoto (2001). "Dibenzofulvene, a 1,1-Diphenylethylene Analogue, Gives a π-Stacked Polymer by Anionic, Free-Radical, and Cationic Catalysts". J. Am. Chem. Soc. 123 (37): 9182–9183. doi:10.1021/ja0111131. PMID 11552835.
- ↑ EP0742190 A1, BASF , 13 Nov 1996, Process for the Preparation of Diarylethanes
 |

