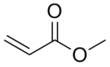
Chemistry:Methyl acrylate
| |||
| Names | |||
|---|---|---|---|
| Preferred IUPAC name
Methyl prop-2-enoate[1] | |||
| Other names | |||
| Identifiers | |||
3D model (JSmol)
|
|||
| ChEMBL | |||
| ChemSpider | |||
| KEGG | |||
PubChem CID
|
|||
| UNII | |||
| |||
| |||
| Properties | |||
| C4H6O2 | |||
| Molar mass | 86.090 g·mol−1 | ||
| Appearance | Colorless liquid | ||
| Odor | Acrid[2] | ||
| Density | 0.95 g/cm3[3] | ||
| Melting point | −74 °C (−101 °F; 199 K)[3] | ||
| Boiling point | 80 °C (176 °F; 353 K)[3] | ||
| 5 g/100 mL | |||
| Vapor pressure | 65 mmHg (20°C)[2] | ||
| Viscosity | |||
| Hazards | |||
| Main hazards | Harmful (Xn); Highly flammable (F+) | ||
| Safety data sheet | Oxford MSDS | ||
| Flash point | −3 °C (27 °F; 270 K)[3] | ||
| Explosive limits | 2.8–25%[2] | ||
| Lethal dose or concentration (LD, LC): | |||
LC50 (median concentration)
|
3575 ppm (mouse) 1350 ppm (rat, 4 hr) 1000 ppm (rat, 4 hr) 2522 ppm (rabbit, 1 hr)[5] | ||
| NIOSH (US health exposure limits): | |||
PEL (Permissible)
|
TWA 10 ppm (35 mg/m3) [skin][2] | ||
REL (Recommended)
|
TWA 10 ppm (35 mg/m3) [skin][2] | ||
IDLH (Immediate danger)
|
250 ppm[2] | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |||
| Infobox references | |||
Methyl acrylate is an organic compound, more accurately the methyl ester of acrylic acid. It is a colourless liquid with a characteristic acrid odor. It is mainly produced to make acrylate fiber, which is used to weave synthetic carpets.[6] It is also a reagent in the synthesis of various pharmaceutical intermediates. Owing to the tendency of methyl acrylate to polymerize, samples typically contain an inhibitor such as hydroquinone.
Production
The standard industrial reaction for producing methyl acrylate is esterification with methanol under acid catalysis (sulfuric acid, p-toluenesulfonic acid or acidic ion exchangers.[7]). The transesterification is facilitated because methanol and methyl acrylate form a low boiling azeotrope (boiling point 62–63 °C).[8]
The patent literature[9] describes a one-pot route involving vapor-phase oxidation of propene or 2-propenal with oxygen in the presence of methanol.
Other methods
Methyl acrylate can be prepared by debromination of methyl 2,3-dibromopropanoate with zinc.[10] Methyl acrylate is formed in good yield on pyrolysis of methyl lactate in the presence of ethenone (ketene).[11] Methyl lactate is a renewable "green chemical". Another patent[12] describes the dehydration of methyl lactate over zeolites.
The nickel tetracarbonyl-catalyzed hydrocarboxylation of acetylene with carbon monoxide in the presence of methanol also yields methyl acrylate.[13] The reaction of methyl formate with acetylene in the presence of transition metal catalysts also leads to methyl acrylate.[14] Both, the alcoholysis of propiolactone with methanol as well as the methanolysis of acrylonitrile via intermediately formed acrylamide sulfate[15] are also proven but obsolete processes.
Use
Methyl acrylate is after butyl acrylate and ethyl acrylate the third most important acrylic ester with a worldwide annual production of about 200,000 tons in 2007.[16] Poly(methyl acrylate) is a tacky material near room temperature, and as such it is not particularly useful as a structural material. Commonly, methyl acrylate (and other acrylate esters) are copolymerized with other alkenes to give useful engineering plastics.[17] A variety of vinyl monomers are used, including styrene and other acrylates.[18] The resulting copolymers give acrylic paints that are harder and more brittle than those with the homologous acrylates. Copolymerizing methyl acrylate with acrylonitrile improves their melt processability to fibers, which could be used as precursors for carbon fibers.[19] Methyl acrylate is the precursor to fibers that are woven to make carpets.
Amino derivatives
Methyl acrylate reacts catalysed by Lewis bases in a Michael addition with amines in high yields to β-alanine derivatives which provide amphoteric surfactants when long-chain amines are used and the ester function is hydrolysed subsequently.
Acrylates are also used in the preparation of poly(amidoamine) (PAMAM) dendrimers typically by Michael addition with a primary amine.
Methyl acrylate is used for the preparation of 2-dimethylaminoethyl acrylate by transesterification with dimethylaminoethanol in significant quantities of over 50,000 tons / year.[20]
Reactions
Methyl acrylate is a classic Michael acceptor, which means that it adds nucleophiles at its terminus. For example, in the presence of a base catalyst, it adds hydrogen sulfide to give the thioether:[21]
- 2 CH2CHCO2CH3 + H2S → S(CH2CH2CO2CH3)2
It is also a good dienophile.
Safety
It is an acute toxin with an -1">50 (rats, oral) of 300 mg/kg and a TLV of 10 ppm.
References
- ↑ 1.0 1.1 "methyl acrylate - Compound Summary". PubChem. https://pubchem.ncbi.nlm.nih.gov/summary/summary.cgi?cid=7294.
- ↑ 2.0 2.1 2.2 2.3 2.4 2.5 NIOSH Pocket Guide to Chemical Hazards. "#0394". National Institute for Occupational Safety and Health (NIOSH). https://www.cdc.gov/niosh/npg/npgd0394.html.
- ↑ 3.0 3.1 3.2 3.3 Record in the GESTIS Substance Database of the Institute for Occupational Safety and Health
- ↑ 4.0 4.1 George, John; Sastry, Nandhibatla V.; Patel, Sunil R.; Valand, Mahendra K. (2002). "Densities, Viscosities, Speeds of Sound, and Relative Permittivities for Methyl Acrylate + 1-Alcohols (C1−C6) atT= (308.15 and 318.15) K". Journal of Chemical & Engineering Data (American Chemical Society (ACS)) 47 (2): 262–269. doi:10.1021/je010268l. ISSN 0021-9568.
- ↑ "Methyl acrylate". Immediately Dangerous to Life and Health Concentrations (IDLH). National Institute for Occupational Safety and Health (NIOSH). https://www.cdc.gov/niosh/idlh/96333.html.
- ↑ Takashi Ohara; Takahisa Sato; Noboru Shimizu; Günter Prescher Helmut Schwind; Otto Weiberg; Klaus Marten; Helmut Greim (2003). "Acrylic Acid and Derivatives". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a01_161.pub2. ISBN 3-527-30673-0.
- ↑ "Esterification: Acrylate esters (MA, EA, BA, MMA, 2-EHA)". amberlyst.com. http://www.amberlyst.com/acrylate.htm.
- ↑ Chessie E. Rehberg (1955). "n-Butyl acrylate". Organic Syntheses 26: 18. http://www.orgsyn.org/demo.aspx?prep=CV3P0146.; Collective Volume, 3, pp. 146
- ↑ Ferlazzo, Natale; Gian Fausto Buzzi & Marcello Ghirga, "Process for the production of methyl acrylate", US patent 3925463, published 1975-12-09, assigned to Societa' Italiana Resine S.I.R., S.p.A.
- ↑ F. Beilstein: Handbuch der organischen Chemie, 3. Auflage, 1. Band. Verlag Leopold Voss, 1893, S. 501. Volltext.
- ↑ Hagemeyer, Hugh J., "Preparation of methyl acrylate", US patent 2417748, published 1947-03-18, assigned to Eastman Kodak Company
- ↑ Abe, Takafumi & Shinichi Hieda, "Process for preparing unsaturated carboxylic acid or ester thereof", US patent 5250729, published 1993-10-05, assigned to Mitsubishi Gas Chemical Company
- ↑ W. Reppe, J. Liebigs Ann. Chem., 582 (1), 116-132 (1953)
- ↑ Liu, Zhao-Tie; Jia-Qi Zhang & Xian-Gui Yang, "Method for synthesizing methyl acrylate", US patent 6022990, published 2000-02-08, assigned to Chengdu Institute of Organic Chemistryand National Research and Engineering Centre for Coal Slurry Gasification and Coal Chemical Industry
- ↑ H.-J. Arpe, Industrielle Organische Chemie, 6. Aufl., Wiley-VCH Verlag, Weinheim, 2007, ISBN 978-3-527-31540-6.
- ↑ CEH Marketing Research Report Acrylic Acid and Esters, SRI Consulting, July 2007.
- ↑ Penzel, Erich; Ballard, Nicholas; Asua, José M. (2018). "Polyacrylates". Ullmann's Encyclopedia of Industrial Chemistry. pp. 1–20. doi:10.1002/14356007.a21_157.pub2. ISBN 978-3-527-30673-2.
- ↑ DOW Methyl acrylate, Product Safety Assessment
- ↑ V. A. Bhanu (2002), "Synthesis and characterization of acrylonitrile methyl acrylate statistical copolymers as melt processable carbon fiber precursors" (in German) (PDF), Polymer 43 (18): 4841–4850, doi:10.1016/S0032-3861(02)00330-0, https://www.researchgate.net/publication/229126444
- ↑ Paul, Jean-Michel; Boris Tonnelier & Francis Augustin, "Composition including dialkyl tin oxide and use thereof as a transesterification catalyst for the synthesis of (meth)acrylic esters", WO patent 2010136696, published 2010-12-02, assigned to Arkema
- ↑ Edward A. Fehnel and Marvin Carmack (1950). "Methyl-β-dipropionate". Organic Syntheses 30: 65. http://www.orgsyn.org/demo.aspx?prep=CV4P0669.; Collective Volume, 4, pp. 669
 |