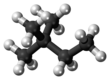
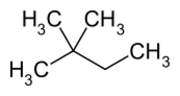
Chemistry:2,2-Dimethylbutane
| |||
|
| |||
| Names | |||
|---|---|---|---|
| Preferred IUPAC name
2,2-Dimethylbutane[2] | |||
| Other names
Neohexane,[1] 22DMB
| |||
| Identifiers | |||
3D model (JSmol)
|
|||
| 1730736 | |||
| ChEMBL | |||
| ChemSpider | |||
| EC Number |
| ||
PubChem CID
|
|||
| RTECS number |
| ||
| UNII | |||
| UN number | 1208 | ||
| |||
| |||
| Properties | |||
| C6H14 | |||
| Molar mass | 86.178 g·mol−1 | ||
| Appearance | Colorless liquid | ||
| Odor | Odorless | ||
| Density | 649 mg mL−1 | ||
| Melting point | −102 to −98 °C; −152 to −145 °F; 171 to 175 K | ||
| Boiling point | 49.7 to 49.9 °C; 121.4 to 121.7 °F; 322.8 to 323.0 K | ||
| log P | 3.51 | ||
| Vapor pressure | 36.88 kPa (at 20 °C) | ||
Henry's law
constant (kH) |
6.5 nmol Pa−1 kg−1 | ||
| -76.24·10−6 cm3/mol | |||
Refractive index (nD)
|
1.369 | ||
| Thermochemistry | |||
Heat capacity (C)
|
189.67 J K−1 mol−1 | ||
Std molar
entropy (S |
272.00 J K−1 mol−1 | ||
Std enthalpy of
formation (ΔfH⦵298) |
−214.4–−212.4 kJ mol−1 | ||
Std enthalpy of
combustion (ΔcH⦵298) |
−4.1494–−4.1476 MJ mol−1 | ||
| Hazards | |||
| GHS pictograms |    
| ||
| GHS Signal word | DANGER | ||
| H225, H304, H315, H336, H411 | |||
| P210, P261, P273, P301+310, P331 | |||
| NFPA 704 (fire diamond) | |||
| Flash point | −29 °C (−20 °F; 244 K) | ||
| 425 °C (797 °F; 698 K) | |||
| Explosive limits | 1.2–7.7% | ||
| NIOSH (US health exposure limits): | |||
PEL (Permissible)
|
none[3] | ||
| Related compounds | |||
Related alkanes
|
|||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |||
| Infobox references | |||
2,2-Dimethylbutane, trivially known as neohexane, is an organic compound with formula C6H14 or (H3C-)3-C-CH2-CH3. It is therefore an alkane, indeed the most compact and branched of the hexane isomers — the only one with a quaternary carbon and a butane (C4) backbone.
Synthesis
2,2-Dimethylbutane can be synthesised by the hydroisomerisation of 2,3-dimethylbutane using an acid catalyst.[4]
It can also be synthesised by isomerization of n-pentane in the presence of a catalyst containing combinations of one or more of palladium, platinum, rhodium and rhenium on a matrix of zeolite, alumina, silicon dioxide or other materials. Such reactions create a mixture of final products including isopentane, n-hexane, 3-methylpentane, 2-methylpentane, 2,3-dimethylbutane and 2,2-dimethylbutane. Since the composition of the final mixture is temperature dependant the desired final component can be obtained choice of catalyst and by combinations of temperature control and distillations.[5][6][7]
Uses
Neohexane is used as an additive in fuels and in the manufacture of agricultural chemicals.[8] It is also used in a number of commercial, automobile and home maintenance products, such as adhesives, electronic contact cleaners and upholstery polish sprays.[9]
In laboratory settings, it is commonly used as a probe molecule in techniques which study the active sites of metal catalysts. Such catalysts are used in hydrogen-deuterium exchange, hydrogenolysis, and isomerization reactions. It is well suited to this purpose as 2,2-dimethylbutane contains both an isobutyl and an ethyl group.[10]
See also
- Methylbutane (isopentane)
- 2-Methylpentane (isohexane)
References
- ↑ Haynes, William M. (2010). Handbook of Chemistry and Physics (91 ed.). Boca Raton, Florida: CRC Press. p. 3-194. ISBN 978-1439820773.
- ↑ "2,2-DIMETHYLBUTANE - Compound Summary". PubChem Compound. USA: National Center for Biotechnology Information. 26 March 2005. Identification and Related Records. https://pubchem.ncbi.nlm.nih.gov/summary/summary.cgi?cid=6403&loc=ec_rcs. Retrieved 9 March 2012.
- ↑ NIOSH Pocket Guide to Chemical Hazards. "#0323". National Institute for Occupational Safety and Health (NIOSH). https://www.cdc.gov/niosh/npg/npgd0323.html.
- ↑ "2,2-dimethylbutane". 18 July 2015. https://pubchem.ncbi.nlm.nih.gov/compound/6403. Retrieved 20 July 2015.
- ↑ Rabo, J. A.; Pickert, P. E.; Mays, R. L. (1961). "Pentane and Hexane Isomerization". Industrial & Engineering Chemistry (American Chemical Society (ACS)) 53 (9): 733–736. doi:10.1021/ie50621a029. ISSN 0019-7866.
- ↑ Den Hartog, A. J.; Rek, P. J. M.; Botman, M. J. P.; De Vreugd, C.; Ponec, V. (1988). "Reactions of 2,2-dimethylbutane on platinum-rhenium/alumina catalysts. Effect of sulfur and chlorine on the selectivity". Langmuir (American Chemical Society (ACS)) 4 (5): 1100–1103. doi:10.1021/la00083a006. ISSN 0743-7463.
- ↑ Brown, Ronald; Kemball, Charles; McDougall, Gordon S. (1995). "Exchange reactions of 2,2-dimethylpentane, 2,2-dimethylbutane and 2,2-dimethylpropane over Pt/SiO2 and Rh/SiO2". Journal of the Chemical Society, Faraday Transactions (Royal Society of Chemistry (RSC)) 91 (7): 1131. doi:10.1039/ft9959101131. ISSN 0956-5000.
- ↑ "Hazardous Substance Fact Sheet - 2,2-Dimethylbutane". June 2008. https://nj.gov/health/eoh/rtkweb/documents/fs/1335.pdf.
- ↑ "2,2-Dimethylbutane" (in en). 2021. https://www.whatsinproducts.com/chemicals/view/1/548/000075-83-2.
- ↑ Burch, R.; Paál, Z. (1994). "The use of 2,2-dimethylbutane (neohexane) as a probe molecule of metal catalysts". Applied Catalysis A: General (Elsevier BV) 114 (1): 9–33. doi:10.1016/0926-860x(94)85106-9. ISSN 0926-860X.
 |