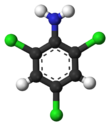
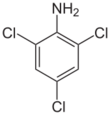
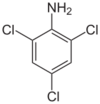
Chemistry:2,4,6-Trichloroaniline
| |||
| Names | |||
|---|---|---|---|
| Preferred IUPAC name
2,4,6-Trichloroaniline | |||
| Identifiers | |||
3D model (JSmol)
|
|||
| ChEMBL | |||
| ChemSpider | |||
| EC Number |
| ||
PubChem CID
|
|||
| UNII | |||
| UN number | 2811 | ||
| |||
| |||
| Properties | |||
| C6H4Cl3N | |||
| Molar mass | 196.46 g·mol−1 | ||
| Appearance | Long needles or fine, light purple fibers [1] | ||
| Melting point | 78.5 °C (173.3 °F; 351.6 K) | ||
| Boiling point | 262 °C (504 °F; 535 K) | ||
| 40 mg/L | |||
| Solubility | chloroform, ether, ethanol [2] | ||
| log P | 3.69 | ||
| Vapor pressure | 1.47×10−7 mmHg | ||
| Acidity (pKa) | 0.07 (for the conjugate acid) | ||
| Basicity (pKb) | 13.93 | ||
| Hazards | |||
| Main hazards | Harmful, corrosive, toxic | ||
| GHS pictograms |    
| ||
| GHS Signal word | Danger | ||
| H301, H311, H317, H331, H373, H410, H411 | |||
| P260, P261, P264, P270, P271, P272, P273, P280, P301+310, P302+352, P304+340, P311, P312, P314, P321, P322, P330, P333+313, P361, P363, P391, P403+233, P405, P501 | |||
| NFPA 704 (fire diamond) | |||
| Flash point | 110 °C (230 °F; 383 K) | ||
| Decomposes | |||
| Lethal dose or concentration (LD, LC): | |||
LD50 (median dose)
|
2400 mg/kg (rat, oral) | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |||
| Infobox references | |||
2,4,6-Trichloroaniline is a chemical compound with a formula of C6H4Cl3N. It is useful as an intermediate in chemical reactions.[2]
Preparation
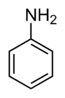
2,4,6-Trichloroaniline can be prepared by reaction of dry aniline with chlorine gas while in an anhydrous solution of carbon tetrachloride. 2,4,6-Trichloroaniline precipitates from solution as a white solid. If water is introduced to the solution the white material will polymerize to form aniline black.[3]
Safety
Occupational exposure to 2,4,6-trichloroaniline may occur through inhalation and dermal contact with this compound at workplaces where 2,4,6-trichloroaniline is produced or used (SRC). The general population may be exposed to 2,4,6-trichloroaniline via drinking water and dermal contact with this compound in dyestuffs, pigments, and pesticides containing 2,4,6-trichloroaniline.[4] 2,4,6-trichloroaniline can be toxic when inhaled or ingested orally. The lethal dose is 2400 mg/kg for a rat.[1]
Upon heating, 2,4,6-trichloroaniline will not undergo combustion, but may release hydrogen chloride, nitrogen oxides or carbon monoxide.[1]
References
- ↑ 1.0 1.1 1.2 "2,4,6-Trichloroaniline(634-93-5) MSDS Melting Point Boiling Point Density Storage Transport". https://www.chemicalbook.com/ProductMSDSDetailCB2854049_EN.htm#5.
- ↑ 2.0 2.1 Pubchem. "2,4,6-Trichloroaniline" (in en). https://pubchem.ncbi.nlm.nih.gov/compound/12471.
- ↑ "Synthesis of 2,4,6-trichloroaniline" (in en-US). 2016-08-15. http://www.prepchem.com/synthesis-246-trichloroaniline/.
- ↑ "TOXNET". https://toxnet.nlm.nih.gov/cgi-bin/sis/search2/r?dbs+hsdb:@term+@rn+@rel+634-93-5.
 |