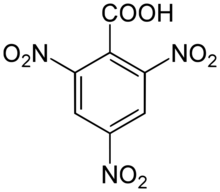
Chemistry:2,4,6-Trinitrobenzoic acid
From HandWiki
Short description: Impact-resistant high explosive
| |
| Names | |
|---|---|
| IUPAC name
2,4,6-Trinitrobenzoic acid
| |
| Identifiers | |
3D model (JSmol)
|
|
| Abbreviations | TNBA |
| ChemSpider | |
| EC Number |
|
PubChem CID
|
|
| UNII | |
| UN number | 0215 |
| |
| |
| Properties | |
| C7H3N3O8 | |
| Molar mass | 257.114 g·mol−1 |
| Appearance | pale yellow |
| Melting point | 228.7 °C (Decomposes) |
| Insoluble | |
| Solubility | Soluble in acetone, methanol, benzene, ethanol, ether |
| log P | 0.23 |
| Vapor pressure | 7.23 10−9mm Hg |
Henry's law
constant (kH) |
2.62 10−14atm cu m/mol |
| Acidity (pKa) | 0.65 |
| Structure | |
| Orthorhombic or rhombohedral | |
| Hazards | |
| Main hazards | explosive |
| GHS pictograms | 
|
| GHS Signal word | Danger |
| H201 | |
| P210, P230, P240, P250, P280, P370+380, P372, P373, P401, P501 | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
2,4,6-Trinitrobenzoic acid (TNBA) is an organic compound with the formula (O2N)3C6H2CO2H. It is a high explosive nitrated derivative of benzoic acid.
Preparation and reactions
2,4,6-Trinitrobenzoic acid is prepared by oxidation of 2,4,6-trinitrotoluene (TNT). It is formed by oxidation of TNT and nitric acid with chlorate[2] and with dichromate.[3]
Upon heating, 2,4,6-trinitrobenzoic acid undergoes decarboxylation to give 1,3,5-trinitrobenzene.[4] Reduction with tin gives 2,4,6-triaminobenzenoic acid, a precursor to phloroglucinol (1,3,5-trihydroxybenzene).[5]
References
- ↑ "2,4,6-Trinitrobenzoic acid" (in en). https://pubchem.ncbi.nlm.nih.gov/compound/8518#section=Safety-and-Hazards.
- ↑ Brown, D. J. (1947). "Improved preparation of 2:4:6-trinitrobenzoic acid" (in en). Journal of the Society of Chemical Industry 66 (5): 168. doi:10.1002/jctb.5000660510. https://onlinelibrary.wiley.com/doi/10.1002/jctb.5000660510.
- ↑ Clarke, H. T.; Hartman, W. W. (1922). "2,4,6-Trinitrobenzoic Acid". Organic Syntheses 2: 95. doi:10.15227/orgsyn.002.0095.
- ↑ Clarke, H. T.; Hartman, W. W. (1922). "1,3,5-Trinitrobenzene". Organic Syntheses 2: 93. doi:10.15227/orgsyn.002.0093.
- ↑ Clarke, H. T.; Hartman, W. W. (1929). "Phloroglucinol". Organic Syntheses 9: 74. doi:10.15227/orgsyn.009.0074.
 |

