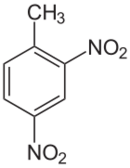
Chemistry:2,4-Dinitrotoluene
| |
| |
| Names | |
|---|---|
| Preferred IUPAC name
1-Methyl-2,4-dinitrobenzene | |
| Other names
Dinitrotoluol, Methyldinitrobenzene
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| KEGG | |
PubChem CID
|
|
| UNII | |
| UN number | Molten: 1600 Solid or liquid: 2038 |
| |
| |
| Properties | |
| C7H6N2O4 | |
| Molar mass | 182.134 g/mol |
| Appearance | Pale yellow to orange crystalline solid |
| Density | 1.52 g/cm3[1] |
| Melting point | 70 °C (158 °F; 343 K)[1] |
| Boiling point | Decomposes at 250–300 °C[1] |
| Vapor pressure | 1.47X10-4 mm Hg @ 22 C |
| Hazards | |
| Main hazards | carcinogen, combustible (though difficult to ignite)[3] |
| Flash point | 207 °C; 404 °F; 480 K |
| Lethal dose or concentration (LD, LC): | |
LD50 (median dose)
|
1,954 mg/kg (oral, mouse)[4] |
LDLo (lowest published)
|
27 mg/kg (cat, oral)[4] |
| NIOSH (US health exposure limits): | |
PEL (Permissible)
|
TWA 1.5 mg/m3 [skin][3] |
REL (Recommended)
|
Ca TWA 1.5 mg/m3 [skin][3] |
IDLH (Immediate danger)
|
Ca [50 mg/m3][3] |
| Explosive data | |
| Shock sensitivity | Insensitive |
| Friction sensitivity | Very low |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
2,4-Dinitrotoluene (DNT) or dinitro is an organic compound with the formula C7H6N2O4. This pale yellow crystalline solid is well known as a precursor to trinitrotoluene (TNT) but is mainly produced as a precursor to toluene diisocyanate.
Isomers of dinitrotoluene
Six positional isomers are possible for dinitrotoluene. The most common one is 2,4-dinitrotoluene. The nitration of toluene gives sequentially mononitrotoluene, DNT, and finally TNT. 2,4-DNT is the principal product from dinitration, the other main product being about 30% 1,3-DN2-T. The nitration of 4-nitrotoluene gives 2,4-DNT.[5]
Applications
Most DNT is used in the production of toluene diisocyanate, which is used to produce flexible polyurethane foams. DNT is hydrogenated to produce 2,4-toluenediamine, which in turn is phosgenated to give toluene diisocyanate. In this way, about 1.4 billion kilograms are produced annually, as of the years 1999–2000.[6] Other uses include the explosives industry. It is not used by itself as an explosive, but some of the production is converted to TNT.
Dinitrotoluene is frequently used as a plasticizer, deterrent coating, and burn rate modifier in propellants (e.g., smokeless gunpowders). As it is carcinogenic[3] and toxic, modern formulations tend to avoid its use. In this application it is often used together with dibutyl phthalate.[citation needed]
Toxicity
Dinitrotoluenes are highly toxic with a threshold limit value (TLV) of 1.5 mg/m3.[7] It converts hemoglobin into methemoglobin.
2,4-Dinitrotoluene is also a listed hazardous waste under 40 CFR 261.24. Its United States Environmental Protection Agency (EPA) Hazardous Waste Number is D030. The maximum concentration that may be contained to not have toxic characteristics is 0.13 mg/L.
References
- ↑ Jump up to: 1.0 1.1 1.2 Record of 2,4-Dinitrotoluene in the GESTIS Substance Database of the Institute for Occupational Safety and Health, accessed on 9. October 2007.
- ↑ Pella, PA. J. Chem. Thermodyn. 9: 301-305, 1977
- ↑ Jump up to: 3.0 3.1 3.2 3.3 3.4 NIOSH Pocket Guide to Chemical Hazards. "#0235". National Institute for Occupational Safety and Health (NIOSH). https://www.cdc.gov/niosh/npg/npgd0235.html.
- ↑ Jump up to: 4.0 4.1 "Dinitrotoluene (mixed isomers)". Immediately Dangerous to Life or Health Concentrations (IDLH). National Institute for Occupational Safety and Health (NIOSH). 4 December 2014. https://www.cdc.gov/niosh/idlh/25321146.html. Retrieved 17 March 2015.
- ↑ Gerald Booth (2007). "Nitro Compounds, Aromatic". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a17_411. ISBN 978-3527306732.
- ↑ Six, C.; Richter, F.. "Ullmann's Encyclopedia of Industrial Chemistry". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a14_611.
- ↑ NIOSH Pocket Guide to Chemical Hazards - Dinitroluene
External links
 |

