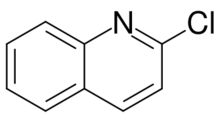
Chemistry:2-Chloroquinoline
From HandWiki
| |
| Names | |
|---|---|
| Preferred IUPAC name
2-Chloroquinoline | |
| Identifiers | |
3D model (JSmol)
|
|
| ChEMBL | |
| ChemSpider | |
| EC Number |
|
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C9H6ClN | |
| Molar mass | 163.60 g·mol−1 |
| Appearance | White solid |
| Melting point | 38 °C (100 °F; 311 K) |
| Boiling point | 266 °C (511 °F; 539 K) |
| Hazards | |
| GHS pictograms | 
|
| GHS Signal word | Warning |
| H315, H319, H335 | |
| P261, P264, P271, P280, P302+352, P304+340, P305+351+338, P312, P321, P332+313, P337+313, P362, P403+233, P405, P501 | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
2-Chloroquinoline is an organic compound with the formula ClC9H6N. It is one of several isomeric chloro derivatives of the bicyclic heterocycle called quinoline. A white solid, 2-chloroquinoline can be prepared from vinylaniline and phosgene.[1] It is a precursor to 2,2'-biquinoline.
References
- ↑ Lee, Byoung Se; Lee, Jae Hak; Chi, Dae Yoon (2002). "Novel Synthesis of 2-Chloroquinolines from 2-Vinylanilines in Nitrile Solvent". Journal of Organic Chemistry 67 (22): 7884–7886. doi:10.1021/jo016196i. PMID 12398521.
 |

