Chemistry:2-Methyl-2-nitrosopropane
| |
| |
| Names | |
|---|---|
| Preferred IUPAC name
2-Methyl-2-nitrosopropane[1] | |
| Other names
tert-Nitrosobutane
| |
| Identifiers | |
3D model (JSmol)
|
|
| Abbreviations | NMP[citation needed] |
| ChemSpider | |
| MeSH | tert-nitrosobutane |
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| C4H9NO | |
| Molar mass | 87.122 g·mol−1 |
| Appearance | Blue liquid |
| Related compounds | |
Related compounds
|
|
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
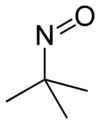
2-Methyl-2-nitrosopropane (MNP or t-nitrosobutane) is the organic compound with the formula (CH3)3CNO. It is a blue liquid that is used in chemical research as a spin trap, i.e. it binds to radicals.
Preparation and structure
t-BuNO is prepared by the following sequence:[2]
- (CH3)3CNH2 → (CH3)3CNO2
- (CH3)3CNO2 → (CH3)3CNHOH
- (CH3)3CNHOH → (CH3)3CNO
The freshly distilled compound is a blue volatile liquid. Like other nitroso compounds, it features a bent C-N=O linkage. Upon standing at room temperature, the blue liquid converts to the colourless solid that is the dimer (m.p. 74-75 °C). In solution, this dimer quickly reverts to the blue monomer.[3]
Reactions
It can be used as a spin trap. This molecule traps unstable free radicals to form stable paramagnetic nitroxide radicals that can be detected and analyzed by electron spin resonance spectroscopy. It is particularly useful for trapping carbon-centered tyrosyl radicals.[4] It has also been used in organic chemistry as electrophile to transform sulfones into aldehydes.[5]
MNP is also an efficient regulator of the radical polymerization of methyl methacrylate through the 'pseudoliving' chain mechanism.[6]
See also
References
- ↑ "tert-nitrosobutane - Compound Summary". PubChem Compound. USA: National Center for Biotechnology Information. 26 March 2005. Identification and Related Records. https://pubchem.ncbi.nlm.nih.gov/summary/summary.cgi?cid=23272&loc=ec_rcs. Retrieved 7 May 2012.
- ↑ Calder, A.; Forrester, A. R.; Hepburn, S. P.. "2-Methyl-2-nitrosopropane and Its Dimer". Organic Syntheses 52: 77. http://www.orgsyn.org/demo.aspx?prep=cv6p0803.; Collective Volume, 6, pp. 803
- ↑ John C. Stowell (1971). "tert-Alkylnitroso compounds. Synthesis and dimerization equilibriums". J. Org. Chem. 36 (20): 3055–3056. doi:10.1021/jo00819a038.
- ↑ David P. Barr; Michael R. Gunther; Leesa J. Deterding; Kenneth B. Tomer; Ronald P. Mason (1996). "ESR Spin-trapping of a Protein-derived Tyrosyl Radical from the Reaction of Cytochrome c with Hydrogen Peroxide". J. Biol. Chem. 271 (26): 15498–15503. doi:10.1074/jbc.271.26.15498. PMID 8663160. Archived from the original on 2008-10-28. https://web.archive.org/web/20081028161706/http://www.jbc.org/cgi/content/abstract/271/26/15498. Retrieved 2009-04-06.
- ↑ Rodrigo, Eduardo; Alonso, Inés; Cid, M. Belén (2018-09-21). "A Protocol To Transform Sulfones into Nitrones and Aldehydes". Organic Letters 20 (18): 5789–5793. doi:10.1021/acs.orglett.8b02483. ISSN 1523-7060. PMID 30207472.
- ↑ Dmitry F Grishin; Lyudmila L Semyonycheva; Elena V Kolyakina (1999). "2-Methyl-2-nitrosopropane as a new regulator of the polymer chain growth". Mendeleev Communications 9 (6): 250–251. doi:10.1070/mc1999v009n06ABEH001161.
 |

