Chemistry:Methyl methacrylate
| |
| |
| Names | |
|---|---|
| Preferred IUPAC name
Methyl 2-methylprop-2-enoate | |
| Other names
Methyl 2-methylpropenoate
methyl methacrylate MMA 2-(methoxycarbonyl)-1-propene | |
| Identifiers | |
3D model (JSmol)
|
|
| 605459 | |
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| EC Number |
|
| 2691 | |
| KEGG | |
PubChem CID
|
|
| RTECS number |
|
| UNII | |
| UN number | 1247 |
| |
| |
| Properties | |
| C5H8O2 | |
| Molar mass | 100.117 g·mol−1 |
| Appearance | Colorless liquid |
| Odor | acrid, fruity[1] |
| Density | 0.94 g/cm3 |
| Melting point | −48 °C (−54 °F; 225 K) |
| Boiling point | 101 °C (214 °F; 374 K) |
| 1.5 g/100 ml | |
| log P | 1.35 [2] |
| Vapor pressure | 29 mmHg (20°C)[1] |
| -57.3·10−6 cm3/mol | |
| Viscosity | 0.6 cP at 20 °C |
| Structure | |
| 1.6–1.97 D | |
| Hazards | |
| Main hazards | Flammable |
| Safety data sheet | Methyl methacrylate MSDS |
| GHS pictograms |  
|
| GHS Signal word | Danger |
| H225, H315, H317, H335 | |
| P210, P233, P240, P241, P242, P243, P261, P264, P271, P272, P280, P302+352, P303+361+353, P304+340, P312, P321, P332+313, P333+313, P362, P363, P370+378, P403+233, P403+235, P405, P501 | |
| Flash point | 2 °C (36 °F; 275 K) |
| 435 °C (815 °F; 708 K) | |
| Explosive limits | 1.7%-8.2%[1] |
| Lethal dose or concentration (LD, LC): | |
LC50 (median concentration)
|
18750 ppm (rat, 4 hr) 4447 ppm (mouse, 2 hr) 3750 ppm (rat) 4808 ppm (mammal)[3] |
LCLo (lowest published)
|
4400 ppm (rat, 8 hr) 4400 ppm (rabbit, 8 hr) 4207 ppm (rabbit, 4.5 hr) 4567 ppm (guinea pig, 5 hr)[3] |
| NIOSH (US health exposure limits): | |
PEL (Permissible)
|
TWA 100 ppm (410 mg/m3)[1] |
REL (Recommended)
|
TWA 100 ppm (410 mg/m3)[1] |
IDLH (Immediate danger)
|
1000 ppm[1] |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
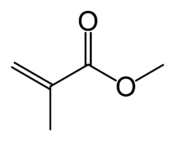
Methyl methacrylate (MMA) is an organic compound with the formula CH2=C(CH3)COOCH3. This colorless liquid, the methyl ester of methacrylic acid (MAA), is a monomer produced on a large scale for the production of poly(methyl methacrylate) (PMMA).[4]
Production and properties
Given the scale of production, many methods have been developed starting from diverse two- to four-carbon precursors.[4][5] Two principal routes appear to be commonly practiced.
Cyanohydrin route
The compound is manufactured by several methods, the principal one being the acetone cyanohydrin (ACH) route. ACH is produced by condensation of acetone and hydrogen cyanide. The cyanohydrin is hydrolyzed in the presence of sulfuric acid to a sulfate ester of the methacrylamide.[6] Methanolysis of this ester gives ammonium bisulfate and MMA. Although widely used, the ACH route coproduces substantial amounts of ammonium sulfate.
- (CH3)2CO + HCN → (CH3)2C(OH)CN
- (CH3)2C(OH)CN + H2SO4 → (CH3)2C(OSO3H)C(O)NH2.
In fact the sulfate ester of the amide is initially produced as an adduct with sulfuric acid ((CH3)2C(OSO3H)C(O)NH2. H2SO4), which is removed in a cracking step. The sulfate ester is then methanolyzed (reacted with methanol):
- (CH3)2C(OSO3H)C(O)NH2 + CH3OH → CH2 =C(CH3)C(O)OCH3 + NH4HSO4
As indicated in the last reaction, each kilogram of methyl methacrylate produced yields roughly 1.1 kg of ammonium hydrogen sulfate. Disposal of this salt is energy intensive. This technology affords more than 3 billion kilograms per year.
The economics of the ACH route have been heavily optimized.[7][8]
Methyl propionate routes
The first stage involves carboalkoxylation of ethylene to produce methyl propionate (MeP):[9]
- C2H4 + CO + CH3OH → CH3CH2CO2CH3
The MeP synthesis is conducted in a continuous-stirred tank reactor at moderate temperature and pressure using proprietary agitation and gas-liquid mixing arrangement.
In a second set of reactions, MeP is condensed with formaldehyde in a single heterogeneous reaction step to form MMA:[10]
- CH3CH2CO2CH3 + CH2O → CH3(CH2)CCO2CH3 + H2O
The reaction of MeP and formaldehyde takes place over a fixed bed of catalyst. This catalyst, caesium oxide on silica, achieves good selectivity to MMA from MeP. The formation of a small amount of heavy, relatively involatile compounds poisons the catalyst. The coke is easily removed and catalyst activity and selectivity restored by controlled, in-situ regeneration. The reactor product stream is separated in a primary distillation so that a crude MMA product stream, free from water, MeP and formaldehyde, is produced. Unreacted MeP and water are recycled via the formaldehyde dehydration process. MMA (>99.9%) is purified by vacuum distillations. The separated streams are returned to the process; there being only a small heavy ester purge stream, which is disposed of in a thermal oxidizer with heat recovered for use in the process.
In 2008, Lucite International commissioned an Alpha MMA plant on Jurong Island in Singapore. This process plant was cheaper to build and run than conventional systems, produces virtually no waste and the feedstocks can even be made from biomass.
Other routes to MMA
Via propionaldehyde
Ethylene is first hydroformylated to give propanal, which is then condensed with formaldehyde to produce methacrolein, The condensation is catalyzed by a secondary amine. Air oxidation of methacrolein to methacrylic acid completes the synthesis of the acid:[7]
- CH3CH2CHO + HCHO → CH2=C(CH3)CHO + H2O
- CH2=C(CH3)CHO + 1⁄2 O2 → CH2=C(CH3)CO2H
From isobutyric acid
As developed by Atochem and Röhm, isobutyric acid is produced by hydrocarboxylation of propene, using HF as a catalyst:
- CH2=CHCH3 + CO + H2O → (CH3)2CHCO2H
Oxidative dehydrogenation of the isobutyric acid yields methacrylic acid. Metal oxides catalyse this process:[7]
- (CH3)2CHCO2H + O → CH2=C(CH3)CO2H + H2O
Methyl acetylene (propyne) process
Using Reppe chemistry, methyl acetylene is converted to MMA. As developed by Shell, this process produces MMA in one step reaction with 99% yield with a catalyst derived from palladium acetate, phosphine ligands, and Bronsted acids as catalyst:[7]
- CH≡CCH3 + CO + CH3OH → CH2=C(CH3)CO2CH3
Isobutylene routes
The reactions by the direct oxidation method consist of two-step oxidation of isobutylene or TBA with air to produce methacrylic acid and esterification by methanol to produce MMA.[7]
- CH2=C(CH3)2 (or (CH3)3C–OH) + O2 → CH2=C(CH3)–CHO + H2O
- CH2=C(CH3)CHO + 1⁄2 O2 → CH2=C(CH3)CO2H
- CH2=C(CH3)CO2H + CH3OH → CH2=C(CH3)CO2CH3 + H2O
A process using isobutylene as a raw material has been commercialized by Escambia Co. Isobutylene is oxidized to provide α-hydroxy isobutyric acid. The conversion uses N2O4 and nitric acid at 5–10 °C in the liquid phase. After esterification and dehydration MMA is obtained. Challenges with this route, aside from yield, involve the handling of large amounts of nitric acid and NOx. This method was discontinued in 1965 after an explosion at an operation plant.[7]
Methacrylonitrile (MAN) process
MAN can be produced by ammoxidation from isobutylene:
- (CH3)2C=CH2 + NH3 + 3⁄2 O2 → CH2=C(CH3)CN + 3 H2O
This step is analogous to the industrial route to acrylonitrile, a related commodity chemical. MAN can be hydrated by sulfuric acid to methacrylamide:
- CH2=C(CH3)CN + H2SO4 + H2O → CH2=C(CH3)–CONH2·H2SO4
- CH2=C(CH3)–CONH2·H2SO4 + CH3OH → CH2=C(CH3)COOCH3 + NH4HSO4
Mitsubishi Gas Chemicals proposed that MAN can be hydrated to methacrylamide without using sulfuric acid and is then esterified to obtain MMA by methylformate.[7]
- CH2=C(CH3)CN + H2O → CH2=C(CH3)–CONH2
- CH2=C(CH3)–CONH2 + HCOOCH3 → CH2=C(CH3)COOCH3 + HCONH2
- HCONH2 → NH3 + CO
Esterification of methacrolein
Asahi Chemical developed a process based on direct oxidative esterification of methacrolein, which does not produce by-products such as ammonium bisulfate. The raw material is tert-butanol, as in the direct oxidation method. In the first step, methacrolein is produced in the same way as in the direct oxidation process by gas phase catalytic oxidation, is simultaneously oxidized and is esterified in liquid methanol to get MMA directly.[7]
- CH2=C(CH3)–CHO + CH3OH + 1⁄2 O2 → CH2=C(CH3)–COOCH3 + H2O
Uses
The principal application, consuming approximately 75% of the MMA, is the manufacture of polymethyl methacrylate acrylic plastics (PMMA). Methyl methacrylate is also used for the production of the co-polymer methyl methacrylate-butadiene-styrene (MBS), used as a modifier for PVC. Another application is as cement used in total hip replacements as well as total knee replacements. Used as the "grout" by orthopedic surgeons to make the bone inserts fix into bone, it greatly reduces post-operative pain from the insertions but has a finite lifespan. Typically the lifespan of methylmethacrylate as bone cement is 20 years before revision surgery is required. Cemented implants are usually only done in elderly populations that require more immediate short term replacements. In younger populations, cementless implants are used because their lifespan is considerably longer.[11] Also used in fracture repair in small exotic animal species using internal fixation.
MMA is a raw material for the manufacture of other methacrylates. These derivatives include ethyl methacrylate (EMA), butyl methacrylate (BMA) and 2-ethyl hexyl methacrylate (2-EHMA). Methacrylic acid (MAA) is used as a chemical intermediate as well as in the manufacture of coating polymers, construction chemicals and textile applications.[12]
Wood can be impregnated with MMA and polymerized in situ to produce a stabilized product.
Environmental issues and health hazards
In terms of the acute toxicity of methyl methacrylate, the LD50 is 7–10 g/kg (oral, rat). It is an irritant to the eyes and can cause redness and pain.[13][14] Irritation of the skin, eye, and nasal cavity has been observed in rodents and rabbits exposed to relatively high concentrations of methyl methacrylate. Methyl methacrylate is a mild skin irritant in humans and has the potential to induce skin sensitization in susceptible individuals.[15][16]
See also
References
- ↑ 1.0 1.1 1.2 1.3 1.4 1.5 NIOSH Pocket Guide to Chemical Hazards. "#0426". National Institute for Occupational Safety and Health (NIOSH). https://www.cdc.gov/niosh/npg/npgd0426.html.
- ↑ "Methyl methacrylate". https://www.chemsrc.com/en/cas/80-62-6_32386.html.
- ↑ 3.0 3.1 "Methyl methacrylate". Immediately Dangerous to Life and Health Concentrations (IDLH). National Institute for Occupational Safety and Health (NIOSH). https://www.cdc.gov/niosh/idlh/80626.html.
- ↑ 4.0 4.1 Bauer, Jr., William (2002). "Ullmann's Encyclopedia of Industrial Chemistry". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a16_441..
- ↑ Darabi Mahboub, Mohammad Jaber; Dubois, Jean-Luc; Cavani, Fabrizio; Rostamizadeh, Mohammad; Patience, Gregory S. (2018). "Catalysis for the synthesis of methacrylic acid and methyl methacrylate". Chemical Society Reviews 47 (20): 7703–7738. doi:10.1039/C8CS00117K. PMID 30211916.
- ↑ Wiley, Richard H.; Waddey, Walter E. (1949). "Methacrylamide". Organic Syntheses 29: 61. doi:10.15227/orgsyn.029.0061.
- ↑ 7.0 7.1 7.2 7.3 7.4 7.5 7.6 7.7 Nagai, Koichi (2001). "New developments in the production of methyl methacrylate". Applied Catalysis A: General 221 (1–2): 367–377. doi:10.1016/S0926-860X(01)00810-9.
- ↑ "New Catalyst for Methyl Methacrylate Process :: News". 22 August 2012. http://www.chemistryviews.org/details/news/2502791/New_Catalyst_for_Methyl_Methacrylate_Process.html.
- ↑ Scott D. Barnicki (2012). "Chapter 10. Synthetic Organic Chemicals". in James A. Kent. Handbook of Industrial Chemistry and Biotechnology (12th ed.). New York: Springer. ISBN 978-1-4614-4259-2.
- ↑ "Archived copy". http://www.ingenia.org.uk/ingenia/issues/issue45/harris.pdf.
- ↑ Nordin, Margareta (2001). Basic Biomechanics of the Musculoskeletal System. New York: Lippincott Williams & Wilkins. pp. 401–419. ISBN 978-0-683-30247-9.
- ↑ "Mpausa - Methacrylates & Why They Are Important". http://www.mpausa.org/about-methacrylates2/.
- ↑ "Methyl methacrylate". NIOSH Pocket Guide to Chemical Hazards. Centers for Disease Control and Prevention. https://www.cdc.gov/niosh/npg/npgd0426.html.
- ↑ "ICSC 0300 - Methyl methacrylate". International Chemical Safety Cards. United Nations International Labour Organization and World Health Organization. https://www.ilo.org/dyn/icsc/showcard.display?p_lang=en&p_card_id=0300&p_version=2.
- ↑ "Concise International Chemical Assessment Document 4 : METHYL METHACRYLATE". https://www.who.int/ipcs/publications/cicad/en/cicad04.pdf.
- ↑ "Archived copy". http://www.cdph.ca.gov/programs/hesis/Documents/mma.pdf.
External links
- Chemical data on Chemicalland
- US Environmental Protection Agency, 1994 data
- Intox Cheminfo data
- Methacrylate Producers Association (MPA)
- National Pollutant Inventory – Methyl methacrylate fact sheet
- CDC – NIOSH Pocket Guide to Chemical Hazards
 |
