Chemistry:2-Methylbut-3-yn-2-ol
From HandWiki
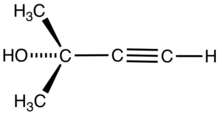
| |
| Names | |
|---|---|
| Preferred IUPAC name
2-Methylbut-3-yn-2-ol | |
Other names
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEMBL | |
| ChemSpider | |
| EC Number |
|
PubChem CID
|
|
| RTECS number |
|
| UNII | |
| UN number | 1987 |
| |
| |
| Properties | |
| C5H8O | |
| Molar mass | 84.118 g·mol−1 |
| Appearance | Colorless liquid |
| Density | 0.8637 g/cm3 |
| Melting point | 3 °C (37 °F; 276 K) |
| Boiling point | 104 °C (219 °F; 377 K) |
| good | |
| Hazards | |
| GHS pictograms |    
|
| GHS Signal word | Danger |
| H225, H226, H302, H315, H318, H319, H335, H361 | |
| P201, P202, P210, P233, P240, P241, P242, P243, P261, P264, P270, P271, P280, P281, P301+312, P302+352, P303+361+353, P304+340, P305+351+338, P308+313, P310, P312, P321, P330, P332+313 | |
| Flash point | 20 °C (68 °F; 293 K) |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
2-Methylbut-3-yn-2-ol is the organic compound with the formula HC2C(OH)Me2 (Me = CH3). A colorless liquid, it is classified as an alkynyl alcohol.
Preparation and use
It arises from the condensation of acetylene and acetone. The addition can be promoted with base[1] (Favorskii reaction) or with Lewis acid catalysts.[2] 2-Methylbut-3-yn-2-ol is produced on an industrial scale as a precursor to terpenes and terpenoids.
2-Methylbut-3-yn-2-ol also is used as a monoprotected version of acetylene. For example, after arylation at carbon, the acetone can be removed with base:[4]
- HC2C(OH)Me2 + ArX + base → ArC2C(OH)Me2 + [Hbase]X
- ArC2C(OH)Me2 → ArC2H + OCMe2
In this regard, 2-methylbut-3-yn-2-ol is used similarly to trimethylsilylacetylene.
References
- ↑ Donald D. Coffman (1940). "Dimethylethynylcarbinol". Org. Synth. 20: 40. doi:10.15227/orgsyn.020.0040.
- ↑ Frantz, Doug E.; Fässler, Roger; Carreira, Erick M. (2000). "Facile Enantioselective Synthesis of Propargylic Alcohols by Direct Addition of Terminal Alkynes to Aldehydes". J. Am. Chem. Soc. 122 (8): 1806-1807. doi:10.1021/ja993838z.
- ↑ Eberhard Breitmaier (2006). Terpenes: Flavors, Fragrances, Pharmaca, Pheromones. Wiley-VCH. doi:10.1002/9783527609949. ISBN 9783527609949.
- ↑ Gordon, John (2001). "2-Methylbut-3-yn-2-ol". e-EROS Encyclopedia of Reagents for Organic Synthesis. pp. 1–2. doi:10.1002/047084289X.rm157. ISBN 0471936235.
 |


