Chemistry:2-Octanone
From HandWiki
| |
| Identifiers | |
|---|---|
3D model (JSmol)
|
|
| ChEBI | |
| EC Number |
|
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| C8H16O | |
| Molar mass | 128.215 g·mol−1 |
| Appearance | colorless liquid |
| Density | 0.820 g/cm3 (20 °C) |
| Melting point | −16 °C (3 °F; 257 K) |
| Boiling point | 172–173 °C (342–343 °F; 445–446 K) |
| Hazards | |
| GHS pictograms | 
|
| GHS Signal word | Warning |
| H226 | |
| P210, P233, P240, P241, P242, P243, P280, P303+361+353, P370+378, P403+235, P501 | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
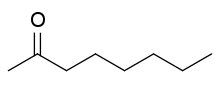
2-Octanone is an organic compound with the formula CH
3C(O)C
6H
13. It is a colorless volatile liquid that is produced commercially for use in the fragrance industry. It is produced by the condensation of acetone and pentanal followed by hydrogenation of the alkene. It can also be produced by selective oxidation of 1-octene.[2] It is one of three octanones, the others being 3-octanone and 4-octanone. It is a common if trace component of many cooked foods.[3]
See also
References
- ↑ "2-Octanone" (in en). https://pubchem.ncbi.nlm.nih.gov/compound/8093#section=Safety-and-Hazards.
- ↑ Siegel, Hardo; Eggersdorfer, Manfred (2000). "Ketones". Ullmann's Encyclopedia of Industrial Chemistry. doi:10.1002/14356007.a15_077. ISBN 9783527306732.
- ↑ Elmore, J. Stephen; Mottram, Donald S.; Enser, Michael; Wood, Jeffrey D. (1999). "Effect of the Polyunsaturated Fatty Acid Composition of Beef Muscle on the Profile of Aroma Volatiles". Journal of Agricultural and Food Chemistry 47 (4): 1619–1625. doi:10.1021/JF980718M. PMID 10564028.
 |
