Chemistry:3-Octanone
From HandWiki
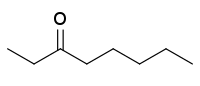
| |
| Names | |
|---|---|
| Preferred IUPAC name
Octan-3-one | |
| Other names
Ethyl amyl ketone; n-Octanone-3; Amyl ethyl ketone; Ethyl pentyl ketone; Ethyl n-amyl ketone; Ethyl n-pentyl ketone
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChemSpider | |
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| C8H16O | |
| Molar mass | 128.215 g·mol−1 |
| Appearance | colorless liquid |
| Density | 0.822 g/mL[1] |
| Boiling point | 167 to 168 °C (333 to 334 °F; 440 to 441 K)[1] |
| insoluble in water[2] | |
| Vapor pressure | 2 mmHg (20°C)[2] |
| Hazards | |
| Flash point | 59 °C; 138 °F; 332 K |
| NIOSH (US health exposure limits): | |
PEL (Permissible)
|
TWA 25 ppm (130 mg/m3)[2] |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Tracking categories (test):
3-Octanone is an organic compound with the formula C
5H
11C(O)C
2H
5. A colorless fragrant liquid, it is classified as a ketone. It is one of three octanones, the others being 2-octanone and 4-octanone.
Occurrence
3-Octanone is found in a variety of sources such as plants (such as lavender),[3] herbs (such as rosemary,[4] basil, and thyme[5]), and nectarines.[6] It was also found to be present in Japanese catnip (Schizonepeta tenuifolia)[7] and the pine king bolete (Boletus pinophilus).[8] It is produced by oyster mushrooms as an insecticide to kill roundworms.[9][10][11]
Uses
3-Octanone is used as a flavor and fragrance ingredient.[12][13][14]
See also
References
- ↑ 1.0 1.1 "3-Octanone". Sigma-Aldrich. http://www.sigmaaldrich.com/catalog/product/aldrich/136913?lang=en.
- ↑ 2.0 2.1 2.2 2.3 NIOSH Pocket Guide to Chemical Hazards. "#0418". National Institute for Occupational Safety and Health (NIOSH). https://www.cdc.gov/niosh/npg/npgd0418.html.
- ↑ Opdyke, D.L.J., ed (1979). Monographs on Fragrance Raw Materials. New York: Pergamon Press. p. 346.
- ↑ Koedam, A. (1978). "Freshly Distilled Oil of the Leaves of Rasmarinus Officianalis L Contained 3-Octanone". Z. Naturforsch. C 33C (1–2): 144. doi:10.1515/znc-1978-1-226.
- ↑ Lee, Seung-Joo; Umano, Katumi; Shibamoto, Takayuki; Lee, Kwang-Geun (2005). "Identification of volatile components in basil (Ocimum basilicum L.) and thyme leaves (Thymus vulgaris L.) and their antioxidant properties". Food Chemistry 91: 131–137. doi:10.1016/j.foodchem.2004.05.056.
- ↑ Takeoka GR (1988). "Nectarine volatiles: vacuum steam distillation versus headspace sampling". J Agric Food Chem 36 (3): 553–560. doi:10.1021/jf00081a037.
- ↑ Yu, S; Chen, Y; Zhang, L; Shan, M; Tang, Y; Ding, A (2011). "Quantitative Comparative Analysis of the Bio-Active and Toxic Constituents of Leaves and Spikes of Schizonepeta tenuifolia at Different Harvesting Times". International Journal of Molecular Sciences 12 (10): 6635–44. doi:10.3390/ijms12106635. PMID 22072908.
- ↑ Bozok, Fuat; Zarifikhosroshahi, Mozhgan; Kafkas, Ebru; Taşkin, Hatira; Buyukalaca, Saadet (2015). "Comparison of Volatile Compounds of Fresh Boletus edulis and B. Pinophilus in Marmara Region of Turkey". Notulae Botanicae Horti Agrobotanici Cluj-Napoca 43: 192–195. doi:10.15835/nbha4319731.
- ↑ Li, Huiping; Liu, Junjie; Hou, Ziqiang; Luo, Xin; Lin, Jinsheng; Jiang, Ning; Hou, Lijuan; Ma, Lin et al. (2022). "Activation of mycelial defense mechanisms in the oyster mushroom Pleurotus ostreatus induced by Tyrophagus putrescentiae". Food Research International 160: 111708. doi:10.1016/j.foodres.2022.111708. PMID 36076457.
- ↑ Li, Huiping; Liu, Junjie; Hou, Ziqiang; Luo, Xin; Lin, Jinsheng; Jiang, Ning; Hou, Lijuan; Ma, Lin et al. (2022). "Activation of mycelial defense mechanisms in the oyster mushroom Pleurotus ostreatus induced by Tyrophagus putrescentiae". Food Research International 160: 111708. doi:10.1016/j.foodres.2022.111708. PMID 36076457.
- ↑ Ouellette, Jennifer (18 January 2023). "Carnivorous oyster mushrooms can kill roundworms with "nerve gas in a lollipop"" (in en-us). https://arstechnica.com/science/2023/01/carnivorous-oyster-mushrooms-can-kill-roundworms-with-nerve-gas-in-a-lollipop/?comments=1&comments-page=1.
- ↑ "3-octanone". thegoodscentscompany.com. http://www.thegoodscentscompany.com/data/rw1004231.html.
- ↑ Ashford RD (1994). Ashford's Dictionary of Industrial Chemicals. London, England: Wavelength Publications Ltd. p. 389.
- ↑ Code of Federal Regulations Title 21
 |

