Chemistry:2-Phosphoglycolate
| Names | |
|---|---|
| IUPAC name
2-phosphonatooxyacetate
| |
| Identifiers | |
| |
3D model (JSmol)
|
|
| ChEBI |
|
| ChemSpider |
|
| EC Number |
|
PubChem CID
|
|
| UNII |
|
| |
| |
| Properties | |
| C2H2O6P−3 | |
| Molar mass | 153.007 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
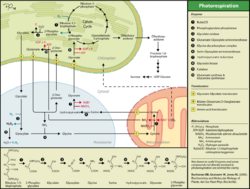
2-Phosphoglycolate (chemical formula C2H2O6P3-; also known as phosphoglycolate, 2-PG, or PG) is a natural metabolic product of the oxygenase reaction mediated by the enzyme ribulose 1,5-bisphosphate carboxylase (RuBisCo).
Synthesis
RuBisCo catalyzes the fixation of atmospheric carbon dioxide in the chloroplasts of plants.[citation needed] It uses ribulose 1,5-bisphosphate (RuBP) as substrate and facilitates carboxylation at the C2 carbon via an endiolate intermediate. The two three-carbon products (3-phosphoglycerate) are subsequently fed into the Calvin cycle. Atmospheric oxygen competes with this reaction. In a process called photorespiration RuBisCo can also catalyze addition of atmospheric oxygen to the C2 carbon of RuBP forming a high energy hydroperoxide intermediate that decomposes into 2-phosphoglycolate and 3-phosphoglycerate.[1] Despite a higher energy barrier for the oxygenation reaction compared to carboxylation, photorespiration accounts for up to 25% of RuBisCo turnover in C3 plants.[2]
Biological role
Plants
In plants, 2-phosphoglycolate has a potentially toxic effect as it inhibits a number of metabolic pathways.[3] The activities of important enzymes in the central carbon metabolism of the chloroplast such as triose-phosphate isomerase, phosphofructokinase, or sedoheptulose 1,7-bisphosphate phosphatase show a significant decrease in the presence of 2-PG. Therefore, degradation of 2-PG during photorespiration is important for cellular homeostasis.
Photorespiration is the main way of chloroplasts to rid themselves of 2-PG.[4] However, this pathway comes at a decreased return on investment ratio as 2-PG is transformed to 3-phosphoglycerate in an elaborate salvage pathway at the cost of one equivalent of NADH and ATP, respectively. In addition, this salvage pathway loses ½ equivalent of previously fixed carbon dioxide and releases ½ equivalent of toxic ammonia per molecule of 2-PG. This leads to a net loss of carbon in photorespiration, making it much less efficient than the Calvin cycle.
However, this salvage pathway can also act as a cellular energy sink, preventing the chloroplastidal electron transport chain from over reduction.[4] It is believed that this pathway also plays a role in improving the abiotic stress response of plants.
Bacteria
2-PG is similarly a toxic product in bacteria. Bacteria remove this substance using a glycerate pathway. This shorter pathway branches out from photorespiration after the formation of glyoxylate, proceeding to use glycoxylate carboxylase and tartronic semialdehyde reductase to rejoin at the formation of glycerate. Some Cyanobacteria can use a combination of photorespiration and glycerate pathways.[5]
Transferring the shorter glycerate pathway into plant chloroplasts, combined with stopping chloroplastic export of glycolate, results in higher photosynthetic efficiency. In tobacco, the biomass increases by 13%, not as good a result as a designed pathway.[6]
Animals
Although mainly produced in plants, 2-PG also plays a role in mammalian metabolism,[3] though the source of 2-PG in mammals remains incompletely understood. It is thought that the processing of breaks in the DNA-strand produces small amounts of 2-PG, but other processes may yield 2-PG as well. The phosphatase subunit of bisphosphoglycerate mutase, an enzyme found in red blood cells, shows an increase in activity by up three orders of magnitude in the presence of 2-PG, resulting in an increase of the oxygen affinity of hemoglobin.
Agricultural significance
RuBisCo has been a potential target for bioengineers for agricultural purposes. A decrease in the oxygenation of RuBP may result in a boost in the efficiency of carbon assimilation in crops such as rice or wheat and therefore increase their net biomass production. Attempts have been made to artificially alter the protein structure of RuBisCo to enhance its catalytic turnover rate. Mutations in the L-subunit of the enzyme, for example, have been shown to increase both the catalytic turnover rate and RuBisCos affinity for carbon dioxide[7]
References
- ↑ Tcherkez, Guillaume (2016). "The mechanism of Rubisco-catalysed oxygenation" (in en). Plant, Cell & Environment 39 (5): 983–997. doi:10.1111/pce.12629. ISSN 1365-3040. PMID 26286702.
- ↑ Zelitch, Israel; Schultes, Neil P.; Peterson, Richard B.; Brown, Patrick; Brutnell, Thomas P. (January 2009). "High Glycolate Oxidase Activity Is Required for Survival of Maize in Normal Air". Plant Physiology 149 (1): 195–204. doi:10.1104/pp.108.128439. ISSN 0032-0889. PMID 18805949.
- ↑ 3.0 3.1 Flügel, Franziska; Timm, Stefan; Arrivault, Stéphanie; Florian, Alexandra; Stitt, Mark; Fernie, Alisdair R.; Bauwe, Hermann (October 2017). "The Photorespiratory Metabolite 2-Phosphoglycolate Regulates Photosynthesis and Starch Accumulation in Arabidopsis". The Plant Cell 29 (10): 2537–2551. doi:10.1105/tpc.17.00256. ISSN 1040-4651. PMID 28947491.
- ↑ 4.0 4.1 Timm, Stefan; Woitschach, Franziska; Heise, Carolin; Hagemann, Martin; Bauwe, Hermann (2019-12-02). "Faster Removal of 2-Phosphoglycolate through Photorespiration Improves Abiotic Stress Tolerance of Arabidopsis". Plants 8 (12): 563. doi:10.3390/plants8120563. ISSN 2223-7747. PMID 31810232.
- ↑ Eisenhut, M; Kahlon, S; Hasse, D; Ewald, R; Lieman-Hurwitz, J; Ogawa, T; Ruth, W; Bauwe, H et al. (September 2006). "The plant-like C2 glycolate cycle and the bacterial-like glycerate pathway cooperate in phosphoglycolate metabolism in cyanobacteria.". Plant Physiology 142 (1): 333–42. doi:10.1104/pp.106.082982. PMID 16877700.
- ↑ "Synthetic glycolate metabolism pathways stimulate crop growth and productivity in the field". Science 363 (6422): eaat9077. January 2019. doi:10.1126/science.aat9077. PMID 30606819.
- ↑ Greene, Dina N.; Whitney, Spencer M.; Matsumura, Ichiro (2007-06-15). "Artificially evolved Synechococcus PCC6301 Rubisco variants exhibit improvements in folding and catalytic efficiency". The Biochemical Journal 404 (Pt 3): 517–524. doi:10.1042/BJ20070071. ISSN 0264-6021. PMID 17391103.
 |