Biology:Bisphosphoglycerate mutase
| bisphosphoglycerate mutase | |||||||||
|---|---|---|---|---|---|---|---|---|---|
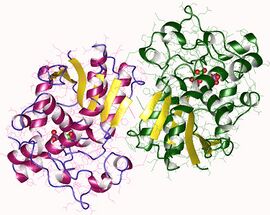 Bisphosphoglycerate mutase homodimer, Human | |||||||||
| Identifiers | |||||||||
| EC number | 5.4.2.4 | ||||||||
| CAS number | 37211-69-1 | ||||||||
| Databases | |||||||||
| IntEnz | IntEnz view | ||||||||
| BRENDA | BRENDA entry | ||||||||
| ExPASy | NiceZyme view | ||||||||
| KEGG | KEGG entry | ||||||||
| MetaCyc | metabolic pathway | ||||||||
| PRIAM | profile | ||||||||
| PDB structures | RCSB PDB PDBe PDBsum | ||||||||
| Gene Ontology | AmiGO / QuickGO | ||||||||
| |||||||||
| 2,3-bisphosphoglycerate mutase | |
|---|---|
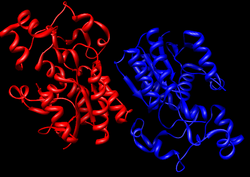 Crystallographic structure of dimeric human bisphosphoglycerate mutase.[1] | |
| Identifiers | |
| Symbol | BPGM |
| NCBI gene | 669 |
| HGNC | 1093 |
| OMIM | 222800 |
| RefSeq | NM_001724 |
| UniProt | P07738 |
| Other data | |
| EC number | 5.4.2.4 |
| Locus | Chr. 7 q31-q34 |
Bisphosphoglycerate mutase (EC 5.4.2.4, BPGM) is an enzyme expressed in erythrocytes and placental cells.[2] It is responsible for the catalytic synthesis of 2,3-Bisphosphoglycerate (2,3-BPG) from 1,3-bisphosphoglycerate. BPGM also has a mutase and a phosphatase function, but these are much less active, in contrast to its glycolytic cousin, phosphoglycerate mutase (PGM), which favors these two functions, but can also catalyze the synthesis of 2,3-BPG to a lesser extent.
Tissue distribution
Because the main function of bisphosphoglycerate mutase is the synthesis of 2,3-BPG, this enzyme is found only in erythrocytes and placental cells.[2] In glycolysis, converting 1,3-BPG to 2,3-BPG would be very inefficient, as it just adds another unnecessary step. Since the main role of 2,3-BPG is to shift the equilibrium of hemoglobin toward the deoxy-state, its production is really only useful in the cells which contain hemoglobin- erythrocytes and placental cells.
Function
1,3-BPG is formed as an intermediate in glycolysis. BPGM then takes this and converts it to 2,3-BPG, which serves an important function in oxygen transport. 2,3-BPG binds with high affinity to Hemoglobin, causing a conformational change that results in the release of oxygen. Local tissues can then pick up the free oxygen. This is also important in the placenta, where fetal and maternal blood come within such close proximity. With the placenta producing 2,3-BPG, a large amount of oxygen is released from nearby maternal hemoglobin, which can then dissociate and bind with fetal hemoglobin, which has a much lower affinity for 2,3-BPG.[2]
Structure
Overall
BPGM is a dimer composed of two identical protein subunits, each with its own active site. Each subunit consists six β-strands, β A-F, and ten α-helices, α 1–10. Dimerization occurs along the faces of β C and α 3 of both monomers.[1] BPGM is roughly 50% identical to its PGM counterpart, with the main active-site residues conserved in nearly all PGMs and BPGMs.[1]
Important residues
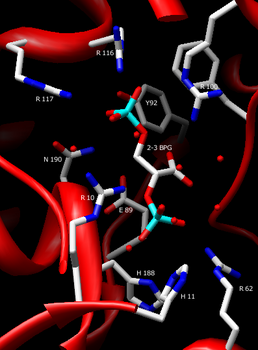
- His11: the nucleophile of the 1,2-BPG to 1,3-BPG reaction. Rotates back and forth with the help of His-188 to get in an in-line position in order to attack the 1’ phosphate group.[3]
- His-188: involved in overall stability of protein,[4] as well as hydrogen bonding to substrate, as His-11, which it pulls into its catalytic position.
- Arg90: although not involved directly in binding, this positively charged residue is essential to overall stability of the protein. Can be substituted with Lysine with little effect on catalysis.[4]
- Cys23: has little effect on overall structure, but large effect on reactivity of the enzyme.[5]
-
2-3 Bisphosphoglycerate in the active site of Bisphosphoglycerate Mutase. Depicted and labeled are the residues that assist in holding the substrate in place: Arg10, Arg62, Arg100, Arg116, Arg117, His11, His188, Tyr92, Asn190, Glu89.
-
2-D depiction of all residues in active site that help hold the substrate in the proper position for mutation.[3]
-
Mechanism for conversion of 1-3BPG to 2-3 BPG.[3]
Mechanism of catalysis
1,3-BPG binds to the active site, which causes a conformational change, in which the cleft around the active site closes in on the substrate, securely locking it in place.[3] 1,3-BPG forms a large number of hydrogen bonds to the surrounding residues, many which are positively charged, severely restricting its mobility. Its rigidity suggests a very enthalpically driven association. Conformational changes cause His11 to rotate, partially aided by hydrogen bonding to His188. His11 is brought in–line with the phosphate group, and then goes through an SN2 mechanism in which His11 is the nucleophile that attacks the phosphate group.[3] The 2’ hydroxy group then attacks the phosphate and removes it from His11, thereby creating 2,3-BPG.
References
- ↑ 1.0 1.1 1.2 PDB: 1T8P; "Crystal structure of human bisphosphoglycerate mutase". J. Biol. Chem. 279 (37): 39132–8. September 2004. doi:10.1074/jbc.M405982200. PMID 15258155.
- ↑ 2.0 2.1 2.2 "Novel placental expression of 2,3-bisphosphoglycerate mutase". Placenta 27 (8): 924–7. August 2006. doi:10.1016/j.placenta.2005.08.010. PMID 16246416.
- ↑ 3.0 3.1 3.2 3.3 3.4 "Seeing the process of histidine phosphorylation in human bisphosphoglycerate mutase". J. Biol. Chem. 281 (51): 39642–8. December 2006. doi:10.1074/jbc.M606421200. PMID 17052986.
- ↑ 4.0 4.1 "Amino acid residues involved in the catalytic site of human erythrocyte bisphosphoglycerate mutase. Functional consequences of substitutions of His10, His187 and Arg89". Eur. J. Biochem. 213 (1): 493–500. April 1993. doi:10.1111/j.1432-1033.1993.tb17786.x. PMID 8477721.
- ↑ "Critical role of human bisphosphoglycerate mutase Cys22 in the phosphatase activator-binding site". J. Biol. Chem. 272 (22): 14045–50. May 1997. doi:10.1074/jbc.272.22.14045. PMID 9162026.
Further reading
- Fujita T (1 December 1998). "Human erythrocyte bisphosphoglycerate mutase: inactivation by glycation in vivo and in vitro". J Biochem 124 (6): 1237–44. doi:10.1093/oxfordjournals.jbchem.a022243. PMID 9832630.
External links
- Bisphosphoglycerate Mutase at the US National Library of Medicine Medical Subject Headings (MeSH)
- EC 5.4.2.4
{{Navbox
| name = Glycolysis enzymes | title = Metabolism: carbohydrate metabolism: [[Biology:Glycoglycolysis/gluconeogenesis enzymes | state = autocollapse | listclass = hlist
| group1 = Glycolysis
| list1 =
| |
| group2 = Gluconeogenesis only| list2 =
| to oxaloacetate: | |
|---|---|
| from lactate (Cori cycle): | |
| from alanine (Alanine cycle): | |
| from glycerol: |
| group4 = Regulatory | list4 =
}}
 |
![2-D depiction of all residues in active site that help hold the substrate in the proper position for mutation.[3]](/wiki/images/thumb/d/da/Coordination_in_Active_site.png/261px-Coordination_in_Active_site.png)
![Mechanism for conversion of 1-3BPG to 2-3 BPG.[3]](/wiki/images/thumb/a/a4/Mechanism_of_1-3BPG_to_2-3BPG.png/300px-Mechanism_of_1-3BPG_to_2-3BPG.png)

