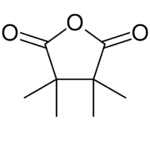
Chemistry:3,3,4,4-Tetramethyltetrahydrofuran-2,5-dione
| |
| Names | |
|---|---|
| Preferred IUPAC name
3,3,4,4-Tetramethyloxolane-2,5-dione | |
| Other names
Tetrahydro-3,3,4,4-tetramethylfuran-2,5-dione; 3,3,4,4-tetramethytetrahydrofuran-2,5-quinone; dihydro-3,3,4,4-tetramethyl-2,5-furandione; tetramethylsuccinic anhydride; TMSA
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| C8H12O3 | |
| Molar mass | 156.181 g·mol−1 |
| Appearance | White crystalline[1] |
| Density | 1.044 g/cm3[2] |
| Melting point | 147 °C (297 °F; 420 K)[1] |
| Boiling point | 226.1 °C (439.0 °F; 499.2 K) 760mmHg[2] |
Refractive index (nD)
|
1.434[2] |
| Hazards | |
| Flash point | 93.2 °C (199.8 °F; 366.3 K)[2] |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
In chemistry, 3,3,4,4-tetramethyltetrahydrofuran-2,5-dione is a heterocyclic compound with the formula C8H12O3, or (CH3)2(COC2COO)(CH3)2. It is a white crystalline solid with a pungent camphoraceous odor.[1]
The compound is also called 3,3,4,4-tetramethyloxolane-2,5-dione (its IUPAC name) or 3,3,4,4-tetramethylsuccinic anhydride,[2] namely the anhydride of 2,2,3,3-tetramethylsuccinic acid, and sometimes abbreviated as TMSA.[3] It can be seen as derivative of tetrahydrofuran-2,5-dione (oxolane-2,5-dione) with two methyl groups replacing two hydrogen atoms on each of the carbon atoms in the ring that are not adjacent to the ring oxygen.[2]
Synthesis and chemistry
The compound is soluble in petroleum ether.[1][4]
The compound was described in 1890 by Karl von Auwers and Victor Meyer who obtained it by thermal decomposition of 2,2,3,3-tetramethylsuccinic acid.[1][5] It can also be obtained, in > 50% yield, from 3,3,4,4-tetramethylpyrrolidine-2,5-dione[4] Other synthesis routes include
- treatment of 2,2'-Azobis(2-methylpropionitrile) with sulfuric acid (1896) [6]
- decomposition of the hydroxy-lactone of 2,2,3,3-tetramethyl-1-one-glutaric acid with release of carbon monoxide (1927) [7]
See also
- 3,3,4,4-Tetramethyltetrahydrofuran
- 2,2,5,5-Tetramethyltetrahydrofuran-3,4-dione
References
- ↑ 1.0 1.1 1.2 1.3 1.4 Karl von Auwers, Victor Meyer (1890), Ueber Tetramethylbernsteinsäure und Trimethylglutarsäure. Berichte der deutschen chemischen Gesellschaft, volume 23, issue 1, pages 293,301,304–305. doi:10.1002/cber.18900230151
- ↑ 2.0 2.1 2.2 2.3 2.4 2.5 "3,3,4,4-tetramethyloxolane-2,5-dione" at Molbase. Accessed on 2015-08-01.
- ↑ Subat Turdi, Peisheng Xu, Qun Li, Youqing Shen, Parhat Kerram, and Jun Ren (2008), Amidization of Doxorubicin Alleviates Doxorubicin-Induced Contractile Dysfunction and Reduced Survival in Murine Cardiomyocytes. Toxicology Letters volume 178, issue 3, pages 197–201. doi:10.1016/j.toxlet.2008.03.010
- ↑ 4.0 4.1 Snezna Bizilj, David P. Kelly, Algirdas K. Serelis, David H. Solomon, Kathleen E. White (1985). The Self-Reactions of 1-Methoxycarbonyl-1-methylethyl and Higher Ester Radicals: Combination vs Disproportionation and Oligomeric Products from Secondary Reactions. Australian Journal of Chemistry, volume 38, issue 11, pages 1657–1673. doi:10.1071/CH9851657
- ↑ Karl von Auwers, O. Ungemach (1935) Zur Zerreißbarkeit der Kohlenstoffkette in Bernsteinsäure-Derivaten. Berichte der deutschen chemischen Gesellschaft, volume 68, pages 23, 349–351. doi:10.1002/cber.19350680228
- ↑ J. Thiele, K. Heuser (1896). Ueber Hydrazinderivate der Isobuttersäure. Justus Liebigs Annalen der Chemie, volume 290, pages 1–43.
- ↑ Eugene Rothstein, Charles William Shoppee (1927), Ring-chain tautomerism. Part XV. The hydroxy-lactone type. Journal of the Chemical Society (UK; Resumed), article LXXVIII, pages 531-534. doi:10.1039/JR9270000531.
 |

