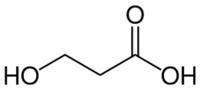
Chemistry:3-Hydroxypropionic acid
| |
| |
| Names | |
|---|---|
| Preferred IUPAC name
3-Hydroxypropanoic acid | |
| Other names
3-Hydroxypropionic acid
Hydracrylic acid Ethylene lactic acid | |
| Identifiers | |
3D model (JSmol)
|
|
| 3DMet | |
| 773806 | |
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| DrugBank | |
| EC Number |
|
| KEGG | |
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| C3H6O3 | |
| Molar mass | 90.08 g/mol |
| Melting point | <25 °C 143 °C (sodium salt) |
| Boiling point | Decomposes |
| Very soluble | |
| Acidity (pKa) | 4.87[2] |
| Related compounds | |
Related carboxylic acids
|
acetic acid glycolic acid propionic acid lactic acid malonic acid butyric acid hydroxybutyric acid |
Related compounds
|
1-propanol 2-propanol propionaldehyde acrolein |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
3-Hydroxypropionic acid is a carboxylic acid, specifically a beta hydroxy acid. It is an acidic viscous liquid with a pKa of 4.9.[2] It is very soluble in water, soluble in ethanol and diethyl ether. Upon distillation, it dehydrates to form acrylic acid, and is occasionally called hydracrylic acid
3-Hydroxypropionic acid is used in the industrial production of various chemicals such as acrylates.
Synthesis
3-Hydroxypropionic acid can be obtained by base-induced hydration of acrylic acid followed by reacidification. Another synthesis involves cyanation of ethylene chlorohydrin followed by hydrolysis of the resulting nitrile. Hydrolysis of propiolactone is yet another route.[3]
Potential applications
The polyester poly(3-hydroxypropionic acid) is a biodegradable polymer.[4] The method combines the high-molecular weight and control aspects of ring-opening polymerization with the commercial availability of the beta hydroxy acid, 3-hydroxypropionic acid which is abbreviated as 3-HP. Since 3-HPA can be derived from biological sources, the resulting material, poly(3-hydroxypropionic acid) or P(3-HPA), is biorenewable.
Genetically encoded 3-hydroxypropionic acid inducible system
3-Hydroxypropionic acid can be produced by engineered microbes.[5]
A genetically encoded 3-hydroxypropionic acid inducible system has been characterized in bacteria demonstrating that such system in combination with fluorescent reporter protein can be utilized as a biosensor to measure intracellular and extracellular 3-HP concentrations by fluorescence output.[6]
See also
- Lactic acid (2-hydroxypropanoic acid)
- listed as hydracrylic acid in the Merck index, 12th Edition
References
- ↑ Merck Index, 11th Edition, 4681.
- ↑ 2.0 2.1 Handbook of Chemistry and Physics, CRC press, 58th edition page D150-151 (1977)
- ↑ Miltenberger, Karlheinz (2000). "Ullmann's Encyclopedia of Industrial Chemistry". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a13_507.
- ↑ "3-HP". http://www.license.umn.edu/Products/Biodegradable-Polyester-Produced-From-Non-toxic--Renewable-Monomer__Z05135.aspx.
- ↑ "Scientists Engineer Extreme Microorganisms to Make Fuel from Atmospheric Carbon Dioxide". 27 March 2013. http://www.popsci.com/science/article/2013-03/scientists-are-engineering-volcanic-microorganisms-chew-co2-spit-back-fuel.
- ↑ Hanko, E.K.R.; Minton, N.P.; Malys, N. (2017). "Characterisation of a 3-hydroxypropionic acid-inducible system from Pseudomonas putida for orthogonal gene expression control in Escherichia coli and Cupriavidus necator". Scientific Reports 7 (1724): 1724. doi:10.1038/s41598-017-01850-w. PMID 28496205. Bibcode: 2017NatSR...7.1724H.
External links
 |

