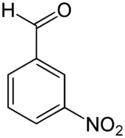
Chemistry:3-Nitrobenzaldehyde
From HandWiki
|
| |||
| Names | |||
|---|---|---|---|
| Preferred IUPAC name
3-Nitrobenzaldehyde | |||
| Other names
m-Nitrobenzaldehyde, meta-nitrobenzaldehyde
| |||
| Properties | |||
| C7H5NO3 | |||
| Molar mass | 151.121 g·mol−1 | ||
| Appearance | Yellowish to brownish crystalline powder or granulate | ||
| Melting point | 58.5 °C (137.3 °F; 331.6 K) | ||
| Boiling point | 164 °C (327 °F; 437 K) at 23 mmHg | ||
| 16.3 mg/mL | |||
| -68.55·10−6 cm3/mol | |||
| Identifiers | |||
3D model (JSmol)
|
|||
| ChEMBL | |||
| ChemSpider | |||
| EC Number |
| ||
PubChem CID
|
|||
| UNII | |||
| |||
| |||
| Hazards | |||
| Main hazards | Harmful,Potentially mutagenic | ||
| GHS pictograms |  
| ||
| GHS Signal word | Warning | ||
| H302, H315, H319, H335, H411 | |||
| P261, P264, P270, P271, P273, P280, P301+312, P302+352, P304+340, P305+351+338, P312, P321, P330, P332+313, P337+313, P362, P391, P403+233, P405, P501 | |||
| NFPA 704 (fire diamond) | |||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |||
| Infobox references | |||
Tracking categories (test):
3-Nitrobenzaldehyde is an organic aromatic compound containing a nitro group meta-substituted to an aldehyde. 3-Nitrobenzaldehyde is the primary product obtained via the mono-nitration of benzaldehyde with nitric acid.
Synthesis
The synthesis of 3-nitrobenzaldehyde is accomplished via nitration of benzaldehyde, which yields mostly the meta-isomer. Product distribution is about 19% for the ortho-, 72% for the meta- and 9% for the para isomers.[3][4]
Uses
3-Nitrobenzaldehyde is a precursor to the drug Tipranavir. It is a mainstay in the synthesis of Dihydropyridine calcium channel blockers. Via selective reduction of the nitro group, it is a precursor to the diazonium salt.[5]
References
- ↑ 3-Nitrobenzaldehyde
- ↑ "3-Nitrobenzaldehyde MSDS". http://www.21cnlab.com/chemdict/MSDS/62565.html.
- ↑ Structure of Benzene, California State University Dominguez Hills
- ↑ Brühne, Friedrich; Wright, Elaine (2011). "Ullmann's Encyclopedia of Industrial Chemistry". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a03_463.pub2.
- ↑ Johannes S. Buck and Walter S. Ide (1933). "m-Chlorobenzaldehyde". Organic Syntheses 13: 28. doi:10.15227/orgsyn.013.0028.
 |