Chemistry:4,4'-Oxydianiline

| |
| Names | |
|---|---|
| Preferred IUPAC name
4,4′-Oxydianiline | |
| Other names
4,4′-Diaminodiphenyl ether; 4-Aminophenyl ether; 4,4′-Oxybisbenzenamine; Bis(4-aminophenyl) ether; 4,4′-ODA
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| EC Number |
|
| KEGG | |
PubChem CID
|
|
| UNII | |
| UN number | 3077 |
| |
| |
| Properties | |
| C12H12N2O | |
| Molar mass | 200.24 g/mol |
| Appearance | Colorless crystalline solid |
| Melting point | 188 to 192 °C (370 to 378 °F; 461 to 465 K) |
| Boiling point | 219 °C (426 °F; 492 K) |
| Insoluble | |
| Hazards | |
| GHS pictograms |   
|
| GHS Signal word | Danger |
| H301, H311, H331, H340, H350, H361f, H411 | |
| P201, P202, P261, P264, P270, P271, P273, P280, P281, P301+310, P302+352, P304+340, P308+313, P311, P312, P321, P322, P330, P361, P363, P391, P403+233, P405, P501 | |
| NFPA 704 (fire diamond) | |
| Flash point | 219 °C (426 °F; 492 K) |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
4,4′-Oxydianiline (ODA) is an organic compound with the formula O(C6H4NH2)2. It is an ether derivative of aniline. This colourless solid is a useful monomer and cross-linking agent for polymers, especially the polyimides, such as Kapton.
Uses
4,4′-Oxydianiline is used in the production of a wide variety of polymer resins. The primary use lies in the production of polyimide and poly(ester)imide resins. These resins are used for their temperature-resistant properties and are utilized in products including wire enamels, coatings, film, adhesives, insulating varnishes, coated fabrics, flame-retardant fibers, oil sealants and retainers, insulation for cables and printed circuits, and laminates and composite for aerospace vehicles.
Other applications of 4,4′-oxydianiline include the production of poly(amide)imide resins (which are used in the manufacture of heat-resistant wire enamels and coatings), as an intermediate in the manufacture of epoxy resins and adhesives, and in the production of aromatic polyether imides.[1] Its use in the production of polyimine vitrimers has also been proposed.[2]
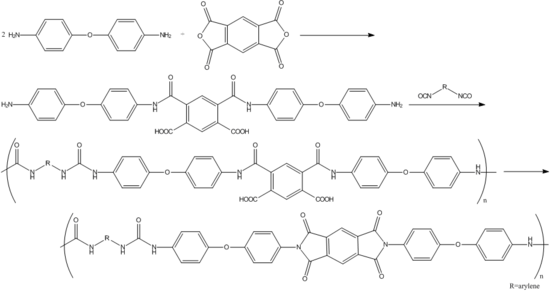
A specific reaction involving industrial use of 4,4′-oxydianiline is in the production of thermostable poly(amideurea) acids, which can be prepared from 4,4′-oxydianiline, pyromellitic dianhydride, and diisocyanates. These poly(amideurea) acids can be used as intermediates in the syntheses of poly(imideurea)s:[3]
Related compounds
References
- ↑ "11th ROC: 4,4'-Oxydianiline". https://ntp.niehs.nih.gov/ntp/roc/content/profiles/oxydianiline.pdf.
- ↑ Schoustra, Sybren K.; Dijksman, Joshua A.; Zuilhof, Han; Smulders, Maarten M. J. (2021). "Molecular control over vitrimer-like mechanics – tuneable dynamic motifs based on the Hammett equation in polyimine materials". Chemical Science 12 (1): 293–302. doi:10.1039/d0sc05458e. ISSN 2041-6520. PMID 34163597.
- ↑ Chiria, C.I; Tanasã, F. (2000). "Polyureas". Ullmann's Encyclopedia of Industrial Chemistry (Weinheim: Wiley-VCH Verlag GmbH & Co. KGaA). doi:10.1002/14356007.d21_d01. ISBN 3527306730.
External links
- MSDS Material Safety Data Sheet provided by Sigma-Aldrich.
 |