Chemistry:4,4'-Methylenedianiline
| |
| Names | |
|---|---|
| Preferred IUPAC name
4,4′-Methylenedianiline | |
| Other names
4,4′-Diaminodiphenylmethane; 4,4′-Methylenebisbenzenamine; MDA; para,para′-Diaminodiphenylmethane; Dianilinomethane; 4,4′-Diphenylmethanediamine; Bis(4-aminophenyl)methane
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| EC Number |
|
| KEGG | |
PubChem CID
|
|
| RTECS number |
|
| UNII | |
| UN number | 2651 |
| |
| |
| Properties | |
| C13H14N2 | |
| Molar mass | 198.269 g·mol−1 |
| Appearance | Colorless solid |
| Odor | faint, amine-like[1] |
| Density | 1.05 g/cm3 (100°C) |
| Melting point | 89 °C (192 °F; 362 K) |
| Boiling point | 398 to 399 °C (748 to 750 °F; 671 to 672 K) |
| 0.125 g/100 ml (20 °C) | |
| Vapor pressure | 0.0000002 mmHg (20°C)[1] |
| Hazards | |
| Main hazards | potential carcinogen[1] |
| GHS pictograms |   
|
| GHS Signal word | Danger |
| H373, H317, H341, H350, H370, H411[2] | |
| P201, P260, P273, P280, P308+313[2] | |
| Flash point | 190 °C; 374 °F; 463 K[1] |
| NIOSH (US health exposure limits): | |
PEL (Permissible)
|
TWA 0.010 ppm ST 0.100 ppm[1] |
REL (Recommended)
|
Ca[1] |
IDLH (Immediate danger)
|
Ca [N.D.][1] |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
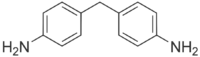
4,4′-Methylenedianiline (MDA) is an organic compound with the formula CH2(C6H4NH2)2. It is a colorless solid, although commercial samples can appear yellow or brown. It is produced on an industrial scale, mainly as a precursor to polyurethanes.
Synthesis and applications
In the industrial production, MDA is produced by reaction of formaldehyde and aniline in the presence of hydrochloric acid.[3]
MDA is a common monomer in the synthesis of polymer materials. These include polyamides,[4] polyimides and polyimines.[5] MDA is also used extensively as a precursor to Methylene diphenyl diisocyanate (MDI). Here, MDA is treated with phosgene to produce MDI. MDI, in turn, is a precursor to many polyurethane foams.[6][7] Lower quantities are used as hardeners in epoxy resins and adhesives, as well as in the production of high-performance polymers.[3] Additionally, hydrogenation of MDA can be performed to produce 4,4,diaminodicyclohexylmethane, which is also used in polymer chemistry.[8]
Safety
MDA is considered a potential occupational carcinogen by the US National Institute for Occupational Safety and Health. The Occupational Safety and Health Administration has set a permissible exposure limit at 0.01 ppm over an eight-hour time-weighted average, and a short-term exposure limit at 0.1 ppm.[9]
It is suspected carcinogen.[6] It is included in the "substances of very high concern" list of the European Chemicals Agency (ECHA).[7] The compound was blamed in a mass poisoning in the vicinity of Epping, Essex, United Kingdom during 1965 during which 84 individuals were poisoned through accidental contamination of flour used to make bread.[10]
Related compounds
References
- ↑ 1.0 1.1 1.2 1.3 1.4 1.5 1.6 NIOSH Pocket Guide to Chemical Hazards. "#0415". National Institute for Occupational Safety and Health (NIOSH). https://www.cdc.gov/niosh/npg/npgd0415.html.
- ↑ 2.0 2.1 Record of 4,4'-Diaminodiphenylmethane in the GESTIS Substance Database of the Institute for Occupational Safety and Health, accessed on 12 February 2021.
- ↑ 3.0 3.1 "Data on manufacture, import, export, uses and release of 4-4' diaminodiphenylmethane". http://echa.europa.eu/doc/consultations/recommendations/tech_reports/tech_rep_mda.pdf.
- ↑ Endo, Taiki; Higashihara, Tomoya (2022). "Direct Synthesis of Thermally Stable Semiaromatic Polyamides by Bulk Polymerization Using Aromatic Diamines and Aliphatic Dicarboxylic Acids". ACS Omega 7 (10): 8753–8758. doi:10.1021/acsomega.1c06983. PMID 35309482.
- ↑ Schoustra, S.K.; De Heer Kloots, M.H.P.; Posthuma, J.; Van Doorn, D.; Dijksman, J.A.; Smulders, M.M.J. (2022). "Raman Spectroscopy Reveals Phase Separation in Imine-Based Covalent Adaptable Networks". Macromolecules 55 (23): 10341–10355. doi:10.1021/acs.macromol.2c01595. PMID 36530523.
- ↑ 6.0 6.1 "ToxFAQs for 4,4'-Methylenedianiline". Agency for Toxic Substances and Disease Registry. https://wwwn.cdc.gov/TSP/ToxFAQs/ToxFAQsLanding.aspx?id=1000&tid=210.
- ↑ 7.0 7.1 "Background document for 4,4'-Diaminodiphenylmethane (MDA)". European Chemicals Agency. http://echa.europa.eu/documents/10162/13640/mda_en.pdf.
- ↑ "Amines, Aliphatic". Ullmann’s Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. 2005. doi:10.1002/14356007.a02_001. ISBN 3527306730.
- ↑ "4,4'-Methylenedianiline". NIOSH Pocket Guide on Chemical Hazards. https://www.cdc.gov/niosh/npg/npgd0415.html.
- ↑ "The Epping jaundice". British Medical Journal 1 (5486): 514–6. February 1966. doi:10.1136/bmj.1.5486.514. PMID 5902696.
External links
- "International Labour Organization". http://www.inchem.org/documents/icsc/icsc/eics1111.htm.
- "NIOSH Pocket Guide to Chemical Hazards". Centers for Disease Control and Prevention. https://www.cdc.gov/niosh/npg/npgd0415.html.
- "European Union Risk Assessment Report". http://ecb.jrc.it/DOCUMENTS/Existing-Chemicals/RISK_ASSESSMENT/REPORT/mdareport008.pdf.
- "An acute case of primary aromatic amines migrating from cooking utensils". Memorandum for the Danish Veterinary and Food Administration on. Danish Institute for Food and Veterinary Research. 12 October 2004. http://www.dfvf.dk/Admin/Public/DWSDownload.aspx?File=Files%2FFiler%2FF%C3%B8devaresikkerhed%2FMaterialer+og+genstande%2FPAA_from_black_nylon_cooking_utentils_2004.pdf.[|permanent dead link|dead link}}]
 |

