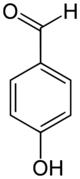
Chemistry:4-Hydroxybenzaldehyde
| |

| |
| Names | |
|---|---|
| Preferred IUPAC name
4-Hydroxybenzaldehyde | |
| Other names
p-Hydroxybenzaldehyde, 4-formylphenol[1]
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| DrugBank | |
| KEGG | |
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| C7H6O2 | |
| Molar mass | 122.123 g·mol−1 |
| Appearance | yellow to tan powder |
| Density | 1.129 g/cm3 (130 °C)[1] |
| Melting point | 116 °C (241 °F; 389 K)[1] |
| Boiling point | 310 to 311 °C (590 to 592 °F; 583 to 584 K) |
| 12.9 g/L[2] | |
| Acidity (pKa) | 7.61 (25 °C)[3] |
| -78.0·10−6 cm3/mol | |
Refractive index (nD)
|
1.57051 (130 °C)[1] |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
4-Hydroxybenzaldehyde (2-hydroxybenzaldehyde) is an organic compound with the formula C
6H
4OH(CHO).[4][5] Along with 3-hydroxybenzaldehyde and 3-hydroxybenzaldehyde, it is one of the three isomers of hydroxybenzaldehyde.
Synthesis, reactions, uses
4-Hydroxybenzaldehyde is prepared by reaction of phenol with chloroform, which gives isomeric hydroxybenzal chlorides. Hydrolysis of the C-Cl bonds gives the aldehyde.[5]
4-Hydroxybenzaldehyde is a precursor to 4-hydroxyphenylglycine, a precursor to penicillins. In the Dakin oxidation, 4-hydroxybenzaldehyde reacts with hydrogen peroxide in base to form hydroquinone.
Metabolism and occurrence
p-Hydroxybenzaldehyde dehydrogenase is an enzyme found in carrots (Daucus carota).[6]
4-Hydroxybenzaldehyde is found in the orchids Gastrodia elata,[7] Galeola faberi,[8] and the Vanilla orchids.
See also
- Salicylaldehyde (2-hydroxybenzaldehyde)
- 3-Hydroxybenzaldehyde
References
- ↑ 1.0 1.1 1.2 1.3 Haynes, p. 3.304
- ↑ Haynes, p. 5.154
- ↑ Haynes, p. 5.92
- ↑ Merck Index, 11th Edition, 8295
- ↑ 5.0 5.1 Maliverney, Christian; Mulhauser, Michel (2000). "Hydroxybenzaldehydes". Kirk-Othmer Encyclopedia of Chemical Technology. doi:10.1002/0471238961.0825041813011209.a01. ISBN 978-0-471-48494-3.
- ↑ Sircar, D.; Mitra, A. (2008). "Evidence for p-hydroxybenzoate formation involving enzymatic phenylpropanoid side-chain cleavage in hairy roots of Daucus carota". Journal of Plant Physiology 165 (4): 407–414. doi:10.1016/j.jplph.2007.05.005. PMID 17658659.
- ↑ Ha, J. H.; Lee, D. U.; Lee, J. T.; Kim, J. S.; Yong, C. S.; Kim, J. A.; Ha, J. S.; Huh, K. (2000). "4-Hydroxybenzaldehyde from Gastrodia elata B1. Is active in the antioxidation and GABAergic neuromodulation of the rat brain". Journal of Ethnopharmacology 73 (1–2): 329–333. doi:10.1016/S0378-8741(00)00313-5. PMID 11025174.
- ↑ Li, Y. M.; Zhou, Z. L.; Hong, Y. F. (1993). "(title in Chinese)" (in zh). Yao Xue Xue Bao = Acta Pharmaceutica Sinica 28 (10): 766–771. PMID 8009989.
Cited sources
- Haynes, William M., ed (2016). CRC Handbook of Chemistry and Physics (97th ed.). CRC Press. ISBN 9781498754293.
 |

