Chemistry:4-Hydroxyphenylglycine
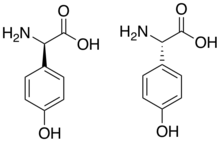 4-Hydroxyphenylglycine (HPG). (R)-D-HPG shown on left; (S)-L-HPG shown on right.
| |
| Names | |
|---|---|
| IUPAC name
Nortyrosine
| |
| Systematic IUPAC name
Amino(4-hydroxyphenyl)acetic acid | |
| Other names
3-Amino-2,3,6-trideoxy-3-methyl-L-lyxo-hexopyranose
| |
| Identifiers | |
3D model (JSmol)
|
|
| 513130 | |
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| EC Number |
|
| KEGG |
|
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| C8H9NO3 | |
| Molar mass | 167.164 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
4-Hydroxyphenylglycine (HPG) is a non-proteogenic amino acid found in vancomycin and related glycopeptides. HPG is synthesized from the shikimic acid pathway and requires four enzymes to synthesize:[1] Both L- and D-HPG are used in the vancomycin class of antibiotics. Tyrosine, a similar amino acid, differs by a methylene group (CH2) between the aromatic ring and the alpha carbon.
Biosynthesis
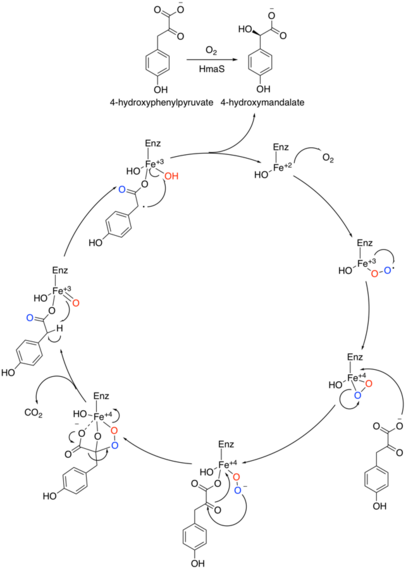
HPG is synthesized from prephenate, an intermediate in the shikimic acid pathway and also a precursor to tyrosine. Prephenate is aromatized by prephenate dehydrogenase (Pdh) using NAD+ as a cofactor to produce 4-hydroxyphenylpyruvate. 4-Hydroxyphenylpyruvate is then oxidized by 4-hydroxymandelate synthase (4HmaS) using oxygen to form 4-hydroxymandelate and hydrogen peroxide. 4HmaS is a non-heme iron-dependent dioxygenase. The reaction mechanism of this unique oxidation was proposed by Choroba et al in 2000[2]
4-Hydroxymandelate is subsequently oxidized by hydroxymandelate oxidase (Hmo) to 4-hydroxylbenzoylformate, using FMN as a cofactor.[3] Finally, 4-hydroxyphenylglycine transaminase (HpgT) transfers an ammonia moiety from a donor to 4-hydroxylbenzoylformate to form HPG. Several different molecules can serve as the nitrogen donor for the transamination, however, Hubbard et al suspect L-tyrosine to serve as the most efficient donor.[4] By doing so, the following cycle is constructed:
HPG is also synthesized in Herpetosiphon aurantiacus using enzymes Haur_(1871,1887,1888).[5]
See also
References
- ↑ Yim, G., Thaker, M. N., Koteva, K., Wright, G. "Glycopeptide antibiotic biosynthesis." The Journal of Antibiotics, 2014, 67, 31-41.
- ↑ Choroba, O. W., Williams, D. H., Spencer, J. B. "Biosynthesis of the Vancomycin Group of Antibiotics: Involvement of an Unusual Dioxygenase in the Pathway to (S)-4-Hydroxyphenylglycine." J. Am. Chem. Soc. 2000, 122, 5389-5390.
- ↑ Li, T.-L., Choroba, O. W., Charles, E. H., Sandercock, A. M., Williams, D. H., Spencer, J. B. "Characterisation of a hydroxymandelate oxidase involved in the biosynthesis of two unusual amino acids occurring in the vancomycin group of antibiotics." Chem. Commun., 2001, 1752-1753.
- ↑ Hubbard, B. K., Thomas, M. G., Walsh, C. T. "Biosynthesis of L-p-hydroxyphenylglycine, a non-proteogenic amino acid constituent of peptide antibiotics." Chemistry & Biology, 2000, 7 (12), 931-942.
- ↑ Kastner, S., Müller, S., Natesan, L., König, G. M., Guthke, R., Nett, M. "4-Hydroxyphenylglycine biosynthesis in Herpetosiphon aurantiacus: a case of gene duplication and catalytic divergence." Arch. Microbiol. 2012, 194, 557-566.
 |