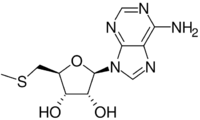
Chemistry:5′-Methylthioadenosine
| |
| Names | |
|---|---|
| IUPAC name
5′-S-Methyl-5′-thioadenosine
| |
| Systematic IUPAC name
(2R,3R,4S,5S)-2-(6-Amino-9H-purin-9-yl)-5-[(methylsulfanyl)methyl]oxolane-3,4-diol | |
Other names
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChemSpider | |
| KEGG | |
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| C11H15N5O3S | |
| Molar mass | 297.33 g·mol−1 |
| Melting point | 205 °C (401 °F; 478 K)[1] |
| Hazards | |
| Lethal dose or concentration (LD, LC): | |
LD50 (median dose)
|
>1000 mg/kg (mouse, oral)[2] |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
5′-Methylthioadenosine is S-methyl derivative of the adenosine. It is an intermediate in the methylthioadenosine (MTA) cycle, also known as the methionine salvage pathway that is universal to aerobic life.[3][4]
Formation
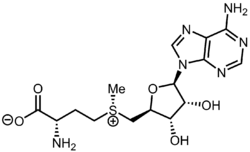
The pervasive cofactor S-adenosyl methionine (SAM) is the precursor to 5′-methylthioadenosine. The sulfonium group in SAM can cleave in three ways, one involves loss of CH2CH2CH(NH3+)CO2−, generating the title compound.
History
In 1912, an adenine nucleoside was isolated by Hunter et al. from yeast that were grown without phosphorus or sulfur.[5] Later in 1925, that substance was shown by Levene and Sobotkal to be adenylthiomethylpentose.[6]
In 1936, Nakahara et al. did experiments on rats that suggested that vitamin L2 deficiency inhibits the ability of female rats for lactation.[7] In 1942, they identified vitamin L2 to be adenylthiomethylpentose.[8] Later studies by Folley et al (1942) refuted Nakahara's claims and demonstrated that L2 is not necessary for lactation and thus L2 is not considered a vitamin today.[9]
Hecht found in 1937 that the body temperature of rabbits, cats and guinea pigs were lowered by 1 to 2 degrees after he gave them adenylthiomethylpentose at a dose of 0.2 g/kg. Kühn et al. replicated this in guinea pigs in 1941.[10]
References
- ↑ Baddiley, J. (1951). "The synthesis of pantothenic acid-2′ and -4′ phosphates as possible degradation products of coenzyme A". Journal of the Chemical Society: 1348–1351. doi:10.1039/JR9510000246.
- ↑ Shimohashi, Hirotaka & Kazuoki Ishihara, "Antiulcer agent", JP patent application H0446124A, published 1992-02-17, assigned to Advance Co. Ltd.
- ↑ Sekowska, A; Ashida, H; Danchin, A (January 2019). "Revisiting the methionine salvage pathway and its paralogues.". Microbial Biotechnology 12 (1): 77–97. doi:10.1111/1751-7915.13324. PMID 30306718.
- ↑ Parveen, Nikhat; Cornell, Kenneth A. (2011). "Methylthioadenosine/S-adenosylhomocysteine nucleosidase, a critical enzyme for bacterial metabolism". Molecular Microbiology 79 (1): 7–20. doi:10.1111/j.1365-2958.2010.07455.x. PMID 21166890.
- ↑ J. A. Mandel u. E. K. Dunham (1912). "Preliminary note on a purine-hexose compound". J. Biol. Chem. 11: 85–86. doi:10.1016/S0021-9258(18)88777-4.
- ↑ P. A. Levene u. H. Sobotka (1925). "The thio-sugar from yeast". J. Biol. Chem. 65 (2): 551–554. doi:10.1016/S0021-9258(18)84864-5. http://www.jbc.org/content/65/2/551.full.pdf.
- ↑ Waro Nakahara; Fumito Inukai; Saburo Ugami (1936). "Factor L2, a Second Dietary Factor for Lactation". Proceedings of the Imperial Academy 12 (9): 289–291. doi:10.2183/pjab1912.12.289.
- ↑ Waro Nakahara; Fumito Inukai; Saburo Ugami (1942). "Adenylthiomethylpentose as a Form of Vitamin L2". Proceedings of the Imperial Academy 18 (8): 477–478. doi:10.2183/pjab1912.18.477. https://www.jstage.jst.go.jp/article/pjab1912/18/8/18_8_477/_pdf.
- ↑ S. J. Folley; K. M. Henry; S. K. Kon (1942). "Lactation and Reproduction on Highly Purified Diets". Nature 150 (3802): 318. doi:10.1038/150318a0. Bibcode: 1942Natur.150Q.318F.
- ↑ R. Kuhn u. K. Henkel (1941). "Über die Senkung der Körpertemperatur durch Adenylthiomethylpentose". Biological Chemistry 269 (1): 41–46. doi:10.1515/bchm2.1941.269.1.41.
Further reading
- Satoh, Kiyoo; Makino, Katashi (1951). "Structure of adenylthiomethylpentose". Nature 167 (4241): 238. doi:10.1038/167238a0. PMID 14806444. Bibcode: 1951Natur.167..238S. Formerly known as vitamin L2.
- Davidson, Michael W. (2018). "Anthranilic acid (vitamin L)". http://micro.magnet.fsu.edu/vitamins/pages/anthranilic.html. Formerly known as vitamin L1.
 |