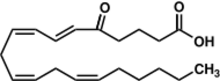
Chemistry:5-oxo-eicosatetraenoic acid
| |
| Names | |
|---|---|
| IUPAC name
5-oxo-6E,8Z,11Z,14Z-eicosatetraenoate
| |
| Other names
5-oxo-ETE, 5-oxoETE, 5-KETE
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| KEGG | |
PubChem CID
|
|
| |
| |
| Properties | |
| C20H30O3 | |
| Molar mass | 318.45 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
5-Oxo-eicosatetraenoic acid (i.e. 5-oxo-6E,8Z,11Z,14Z-eicosatetraenoic acid; also termed 5-oxo-ETE and 5-oxoETE) is a Nonclassic eicosanoid metabolite of arachidonic acid and the most potent naturally occurring member of the 5-HETE family of cell signaling agents. Like other cell signaling agents, 5-oxo-ETE is made by a cell and then feeds back to stimulate its parent cell (see Autocrine signaling) and/or exits this cell to stimulate nearby cells (see paracrine signaling). 5-Oxo-ETE can stimulate various cell types particularly human leukocytes but possesses its highest potency and power in stimulating the human eosinophil type of leukocyte. It is therefore suggested to be formed during and to be an important contributor to the formation and progression of eosinophil-based allergic reactions;[1][2] it is also suggested that 5-oxo-ETE may also contribute inflammation, cancer cell growth, and other pathophysiological responses.[1][3]
Biochemistry and production
In the most common means for its production, cells make 5-oxo-ETE in a four step pathway that involves their stimulus-induced activation of the following pathway: a) the release of arachidonic acid (i.e. 5Z,8Z,11Z,14Z-eicosatetraenoic acid) from its storage sites in membrane phospholipids due to the activation of phospholipase A2 enzymes; b) oxygenation of this arachidonic acid with by activated arachidonate 5-lipoxygenase (ALOX5) to form 5(S)-hydroperoxy-6E,8Z,11Z,14Z-eicosatetraenoic acid (5(S)-HpETE); c) reduction of this 5(S)-HpETE by ubiquitous cellular peroxidases to form 5(S)-hydroxy-6E,8Z,11Z,14Z-eicosatetraenoic acid (5(S)-HETE); and (d) the oxidation of 5(S)-HETE by a microsome-bound nicotinamide adenine dinucleotide phosphate (NADP+)-dependent dehydrogenase enzyme viz., (5-Hydroxyeicosanoid dehydrogenase or 5-HEDH) to form 5-oxo-ETE:[1]
5-HEDH has little or no ability to metabolize the R stereoisomer of 5(S)-HETE viz., 5(R)-HETE, to 5-oxo-ETE. Furthermore, it acts in a fully reversible manner, readily converting 5-oxo-ETE back to 5(S)-HETE. Since cells typically maintain very high levels of NADPH compared to their NADP+ levels, they generally have little or no ability to convert 5(S)-HEE to 5-oxo-ETE, and when confronted with 5-oxo-ETE rapidly metabolize it to 5(S)-HETE.[1] However, cells undergoing aging, senescence, apoptosis, oxidative stress, or other conditions that raise their levels of reactive oxygen species (e.g. superoxide anion, oxygen radicals, and peroxides) either physiologically (e.g. human phagocytes engulfing bacteria) or pathologically (e.g. oxidatively challenged B-lymphocytes) use up NADP+, have low NADPH/NADP+ ratios, and therefore readily convert 5(S)-HETE to 5-oxo-ETE.[1] Thus, many pathological conditions oxidative stress such as occurs in rapidly growing cancers may be important promoters of 5-oxo-ETE accumulation in vivo.
5-Oxo-ETE can also be made form either 5(S)-HpETE (and possibly 5(R)-HpEPE) by the action of cytochrome P450 (CYP) enzymes such as CYP1A1, CYP1A2, CYP1B1, and CYP2S1.[4] from 5(S)-HETE (and probably 5(R)-HETE) by the non-enzymatic attack with heme or various other dehydrating agents;[1] It may also form by the conversion of 5-(S)-HpETE or 5(R)-HpETE to 5-oxo-ETE due to the action of a mouse macrophage 50-60 kilodalton cytosolic protein.[5] The contribution of the latter three pathways to the physiological production of 5-oxo-ETE has not been fully evaluated.
An isomer of 5-oxo-ETE, 5-oxo-(7E,9E,11Z,14Z)-eicosatetraenoic acid, forms non-enzymatically as a byproduct of hydrolyses of the 5-lipooxgenase metabolite, Leukotriene A4. This byproduct differs from 5-oxo-ETE not only in the position and geometry of its double bounds but also in its activity: it stimulates human neutrophils apparently by acting on one or more LTB4 receptors rather than OXER1.[1][6]
Tissue sources
Cellular production
Human neutrophils, monocytes, eosinophils, B-lymphocytes, dendritic cell, platelets, airway epithelial cells and smooth muscle cells, vascular endothelial cells, and skin keratinocytes have been found and/or suggested to make 5-oxo-ETE from endogenous or exogenous 5-HETE, particularly under conditions of oxidative stress; cell lines derived from human cancers such as those from breast, prostate, lung, colon, and various types of leukemia have likewise been shown to be producers of 5-oxo-ETE.[3]
Transcellular production
Cells of one type may release the 5(S)-HETE that they make to nearby cells of a second type which then oxidize the 5(S)-HETE to 5-oxo-ETE. This transcellular production typically involves the limited variety of cell types that express active 5-lipoxygenase, lack HEDH activity because of their high levels of NADPH compared to NADP+ levels, and therefore accumulate 5(S)-HETE, not 5-oxo-ETE, upon stimulation; this 5(S)-ETE can leaves these cells, enter various cell types that possess 5-HEDH activity along with lower NADPH to NADP+ levels, and thereby is converted to 5-oxo-ETE. This transcellular production of 5-oxo-eicosatetraenoates has been demonstrated in vitro with human neutrophils as the 5(S)-HETE producing cells and human PC-3 prostate cancer cells, platelets, and monocyte-derived dendritic cells as the oxidizing cells.[3][7] It is theorized that this transcellular metabolism occurs in vivo and provides a mechanism for controlling 5-oxo-ETE production by allowing it to occur or be augmented at sites were 5-lipoxygenase-containg cells congregate with cell types possessing 5-HEDH and favorable NADPH/NADP+ ratios; such sites, it is theorized, might include those involving allergy, inflammation, oxidative stress, and rapidly growing cancers.[1][3]
Metabolism
As indicated in the previous section, 5-oxo-ETE is readily converted to 5(S)-HETE by 5-HEDH in cells containing very low NADPH/NADP+ ratios. Human neutrophils, an important model cell for investigating 5-oxo-ETE production, take up 5-oxo-ETE and reduce it to 5(S)-HETE; they also form appreciable amounts of 5(S),20-dihydroxy-ETE and small amounts of 5-oxo,20-hydroxy-ETE probably by the action of the ω-hydroxylase cytochrome P450 enzyme, CYP453A on 5(S)-HETE and 5-oxo-ETE, respectively.[3][8] The cells also incorporate the 5(S)-HETE product of 5-oxo-ETE but little or no 5-oxo-ETE itself as an ester into their various phospholipid and glycerolipid pools; however, isolated neutrophil plasma membranes, which lack appreciable 5-HEDH activity, do esterify 5-oxo-ETE into these lipid pools.[1][8]
Several other pathways can metabolize 5-oxo-ETE. First, human eosinophils use Arachidonate 15-lipoxygenase-1 (or possibly Arachidonate 15-lipoxygenase-2 to metabolize 5-oxo-ETE to 5-oxo-15-(S)-hydroperoxy-ETE which is rapidly reduced to 5-oxo-15(S)-hydroxy-ETE; 5-oxo-15(S)-hydroxyl-ETE is about one-third as potent as 5-oxo-ETE in stimulating cells.[1][3] Second, human platelets use 12-lipoxygenase to metabolize 5-oxo-ETE to 5-oxo-12(S)-hydroperxy-eicosatetraenoat which is rapidly converted to 5-oxo-12(S)-hydroxy-eicosatetraenoate (5-oxo-12)S)-hydroxy-ETE); 5-oxo-12(S)-hydroxyl-ETE is a weak antagonist of 5-oxo-ETE.[3] Third, mouse macrophages use a) a cytochrome P450 enzyme to metabolize 5-oxo-ETE to 5-oxo-18-hydroxy-ETE (5-oxo-18-HETE) which is either attacked by a 5-keto-reductase (possibly 5-HEDH) to form 5,18-dihydroxy-eicosatetraenoic acid (5,18-diHETE) or by a Δ6-reductase to form 5-oxo-18-hydroxy-eicosatrienoic acid (5-oxo-18-HETrE) which is then reduced by a 5-keto-reductase (possibly 5-HEDH) to 5,18-dihydroxy-eicosatetrienoic acid (5,18-diHETrE); b) a cytochrome P450 enzyme converts 5-oxo-ETE to 5-oxo-19-hydroxy-eicosatetraenoic acid (5-oxo-19-HETE) which is then either reduced by a keto reductase (possibly 5-HEDH) to 5,19-dihydroxy-eicosatetraenoic acid (5,19-diHETE) or by a Δ6 reductase to 5-oxo-19-hydroxy-eicosatrienoic acid (5-oxo-19-HETrE);[9] or c) leukotriene C4 synthase to metabolize 5-oxo-ETE to 5-oxo-7-glutathionyl-8,11,14-eicosatrienoic acid (FOG7). FOG7 simulates cells by a different mechanism than 5-oxo-ETE; the biological activity of the other mouse-derived metabolites has not been reported.[10][11]
Mechanism of action
The OXER1 receptor
Studies in human neutrophils first detected a plasma membrane-localized site which reversibly bound 5-oxo-ETE and had the attributes of a Gi alpha subunit-linked G protein-coupled receptor based on the ability of 5-oxo-ETE to activate this class of membrane G proteins by a pertussis toxin-sensitive mechanism.[3][8] Subsequently, this receptor was cloned by several groups who termed it the oxoeicosanoid receptor 1 (OXER1), OXE, OXE-R, hGPCR48, HGPCR48, or R527 (its gene is termed OXE1 or OXER1), and found it coupled with and activated the G protein complex composed of the Gi alpha subunit (Gαi) and G beta-gamma complex (Gβγ).[1][3][12] Based on the presence of its mRNA, the OXER1 mRNA is highly expressed in human blood eosinophils, neutrophils, spleen, lung, liver and kidney and at lower levels in human basophils, monocytes, lung macrophages, various cancer cell lines, and an adrenocortical cell line.[1] Orthologs of OXER1 are found in various mammalian species including cats and opossums as well as several species of fish; however, mice and rats lack a clear ortholog of OXER1.[2][3] When bound by 5-oxo-ETE or other 5-HETE family member, OXER1 triggers this G protein complex to dissociate into its Gαi and Gβγ components, with Gβγ being responsible for activating many of the signal pathways that lead to the cellular functional responses elicited by the 5-HETE family of agonists.[13] The cell-activation pathways stimulated by OXER1 include those evoking rises in cytosolic calcium ion levels as well as those activating MAPK/ERK, p38 mitogen-activated protein kinases, cytosolic Phospholipase A2, PI3K/Akt, protein kinase C beta (PKCβ), and (PKCε).[1][3][12][14] Most actions of the 5-HETE family of agonists appear mediated by OXER1. Some of their actions, however, appear to be OXER1-independent, as indicated below.
Other GPCR receptors
Mouse MA-10 cells respond to 5-oxo-ETE but lack OXER1. It has been suggested that these cells' responses to 5-oxo-ETE are mediated by an ortholog to OXER1, mouse niacin receptor 1, Niacr1, which is a G protein-coupled receptor for niacin, or, alternatively, by one or more of the mouse hydroxycarboxylic acid (HCA) family of the G protein-coupled receptors, HCA1 (GPR81), HCA2 (GPR109A), and HCA3 (GPR109B), which are G protein-coupled receptors for fatty acids.[3][15]
PPARγ
5-Oxo-ETE and 5-oxo-15(S)-hydroxy-ETE but not 5-hydroxy members of the 5-HETE family such as 5-(S)-HETE activate peroxisome proliferator-activated receptor gamma (PPARγ). This activation does not proceed through OXER1; rather, it involves the direct binding of the oxo analog to PPARγ with 5-oxo-15-(S)-hydroxy-ETE being more potent than 5-oxo-ETE in binding and activating PPARγ.[16] The Activation of OXER1 receptor and PPARγ by the oxo analogs can have opposing effects on cell function. For example, 5-oxo-ETE-bound OXER1 stimulates whereas 5-oxo-ETE-bound PPARγ inhibits the proliferation of various types of human cancer cell lines; this results in 5-oxo-ETE and 5-oxo-15-(S)-HETE having considerably less potency than anticipated in stimulating these cancer cells to proliferate relative to the potency of 5-(S)-HETE, a relationship not closely following the potencies of these three compounds in activating OXER1.[3][16]
Other mechanisms
5-Oxo-ETE relaxes pre-contracted human bronchi by a mechanism that does not appear to involve OXER1 but is otherwise undefined.[3][17]
Target cells
Inflammatory cells
5-Oxo-ETE is a potent in vitro stimulator and/or enhancer of chemotaxis (i.e. directional migration) and, depending on the cell type, various other responses such as degranulation (i.e. release of granule-bound enzymes), oxidative metabolism (i.e. generation of reactive oxygen species), and production of mediators such as various arachidonic acid metabolites and platelet-activating factor in human eosinophils, basophils, neutrophils, and monocytes.[3][18] Furthermore, the injection of 5-oxo-ETE into the skin of humans causes the local accumulation of circulating blood cells, particularly eosinophils but also to lesser extents neutrophils and monocyte-derived macrophages.[19] The activity of 5-oxo-ETE on the two cell types known to be involved in allergy-based inflammation, eosinophils and basophils, suggests that it may be involved in promoting allergic reactions possibly by attracting through chemotaxis these cells to nascent sites of allergy and/or through stimulating these cells to release granule-bound enzymes, reactive oxygen species, or other promoters of allergic reactions.[3][12] 5-Oxo-ETE's activity on human cells involved in non-allergic inflammatory diseases viz., neutrophils and monocytes, as well as its ability to attack these cell types to the skin of humans suggest that 5-oxo-ETE may also be involved in the broad category of non-allergic inflammatory diseases including those involving host defense against pathogens.[12]
Lung airway smooth muscle cells
5-Oxo-ETE contracts smooth muscle and organ-cultured bronchi isolated from guinea pigs but relaxes bronchi isolated from human lung; the relaxation of human bronchi caused by 5-oxo-ETE may not involve its OXER1.[3][20] These results suggest that 5-oxo-ETE is not directly involved in the bronchoconstriction) that occurs in eosinophil-based allergic asthma reactions in humans.
Cancer cells
5-Oxo-ETE (or other 5-HETE family member) stimulates the growth and/or survival of human cell lines derived from cancers of the prostate, breast, lung, ovary, colon and pancreas[1][3][16][21] These preclinical studies suggest that 5-oxo-ETE (or other 5-HETE family member) may contribute to the cited cancers progression in humans.
Steroidogenic cells
5-oxo-ETE stimulates human H295R adrenocortical cells to increase transcription of steroidogenic acute regulatory protein messenger RNA and produce aldosterone and progesterone by an apparent OXER1-dependent pathway.[15]
Other cell types
5-Oxo-ETE induces an isotonic volume reduction in guinea pig intestinal crypt epithelial cells.[22]
Interaction with other stimuli
5-Oxo-ETE and another potential mediator of human allergic reactions, platelet-activating factor, act in synergy in stimulating human eosinophils and neutrophils: the combined agents elicit responses that are greater than the simple sum of their individual actions and do so at relatively low.[23][24] 5-Oxo-ETE also greatly increases the potencies of complement component 5a, LTB4, and FMLP in stimulating human eosinophils to degranulate and its degranulating activity is greatly increase by pretreating human eosinophils with granulocyte-macrophage colony stimulating factor or human neutrophils with either the latter cytokine or with granulocyte colony-stimulating factor, tumor necrosis factor α, or various nucleotides including ATP.[23][24][25][26] Pretreament of eosinophils with interleukin 5 (a key mediator in eosinophil activation) also increases their in vitro chemotactic response to 5-oxo-ETE.[27] 5-Oxo-ETE also acts in synergy with two chemokines, CCL2 and CCL8, in stimulating monocyte chemotaxis.[18] The interactions of 5-oxo-ETE with these mediators of allergy (e.g. platelet-activating factor, interleukin 5) in eosinophils further suggests that it plays a role in allergic diseases while its interactions with mediators of inflammatory reactions (e.g. tumor necrosis factor α, the colony stimulating factors, and the two CCL chemokines) in neutrophils and monocytes further suggest that it plays a role in inflammatory responses and host defense mechanisms.
Clinical significance
Essentially all of the studies on 5-oxo-ETE's activities and target cells, similar to those on other members of the 5(S)-HETE family of agonists, are best classified as pre-clinical development studies: they have not yet been determined to be important in human pathophysiology. Translation studies are needed to learn if the preclinical studies implicating 5-Oxo-ETE and other 5(S)-HETE family members in allergic diseases, inflammatory diseases, cancer, steroid production, bone remodeling, parturition, and other pathophysiological events, as outlined here and on the 5-HETE page, are relevant to humans and therefore of clinical significance.
See also
- ALOX15
- 5-Hydroxyeicosanoid dehydrogenase
- 5-HETE
- OXER1
References
- ↑ 1.00 1.01 1.02 1.03 1.04 1.05 1.06 1.07 1.08 1.09 1.10 1.11 1.12 1.13 "Biosynthesis, biological effects, and receptors of hydroxyeicosatetraenoic acids (HETEs) and oxoeicosatetraenoic acids (oxo-ETEs) derived from arachidonic acid". Biochimica et Biophysica Acta 1851 (4): 340–55. April 2015. doi:10.1016/j.bbalip.2014.10.008. PMID 25449650.
- ↑ 2.0 2.1 "Biosynthesis and actions of 5-oxoeicosatetraenoic acid (5-oxo-ETE) on feline granulocytes". Biochemical Pharmacology 96 (3): 247–55. August 2015. doi:10.1016/j.bcp.2015.05.009. PMID 26032638.
- ↑ 3.00 3.01 3.02 3.03 3.04 3.05 3.06 3.07 3.08 3.09 3.10 3.11 3.12 3.13 3.14 3.15 3.16 3.17 "The eosinophil chemoattractant 5-oxo-ETE and the OXE receptor". Progress in Lipid Research 52 (4): 651–65. October 2013. doi:10.1016/j.plipres.2013.09.001. PMID 24056189.
- ↑ "Human CYP2S1 metabolizes cyclooxygenase- and lipoxygenase-derived eicosanoids". Drug Metabolism and Disposition 39 (2): 180–90. February 2011. doi:10.1124/dmd.110.035121. PMID 21068195.
- ↑ "Biosynthesis of 5-oxo-6,8,11,14-eicosatetraenoic acid from 5-hydroperoxyeicosatetraenoic acid in the murine macrophage". The Journal of Biological Chemistry 278 (13): 11190–6. March 2003. doi:10.1074/jbc.M208496200. PMID 12547823.
- ↑ "LTA(4)-derived 5-oxo-eicosatetraenoic acid: pH-dependent formation and interaction with the LTB(4) receptor of human polymorphonuclear leukocytes". Biochimica et Biophysica Acta 1484 (1): 51–8. February 2000. doi:10.1016/s1388-1981(99)00198-5. PMID 10685030.
- ↑ "Human dendritic cells are a physiological source of the chemotactic arachidonic acid metabolite 5-oxo-eicosatetraenoic acid". Inflammation Research 49 (11): 633–8. November 2000. doi:10.1007/s000110050641. PMID 11131304.
- ↑ 8.0 8.1 8.2 "Receptors for the 5-oxo class of eicosanoids in neutrophils". The Journal of Biological Chemistry 273 (49): 32535–41. December 1998. doi:10.1074/jbc.273.49.32535. PMID 9829988.
- ↑ "Synthesis of 5-oxo-6,8,11,14-eicosatetraenoic acid and identification of novel omega-oxidized metabolites in the mouse macrophage". The Journal of Pharmacology and Experimental Therapeutics 296 (2): 293–305. February 2001. PMID 11160610.
- ↑ "Glutathione adducts of oxyeicosanoids". Prostaglandins Other Lipid Mediat. 68–69: 471–82. 2002. doi:10.1016/s0090-6980(02)00049-7. PMID 12432937.
- ↑ "Synthesis of 5-oxo-6,8,11,14-eicosatetraenoic acid and identification of novel omega-oxidized metabolites in the mouse macrophage.". J Pharmacol Exp Ther 296 (2): 293–305. Feb 2001. PMID 11160610.
- ↑ 12.0 12.1 12.2 12.3 "International Union of Pharmacology XLIV. Nomenclature for the oxoeicosanoid receptor". Pharmacological Reviews 56 (1): 149–57. March 2004. doi:10.1124/pr.56.1.4. PMID 15001665.W
- ↑ "A biased non-Gαi OXE-R antagonist demonstrates that Gαi protein subunit is not directly involved in neutrophil, eosinophil, and monocyte activation by 5-oxo-ETE". Journal of Immunology 192 (10): 4774–82. May 2014. doi:10.4049/jimmunol.1302013. PMID 24733850.
- ↑ Rossi, A. G.; O'Flaherty, J. T. (1991). "Bioactions of 5-hydroxyicosatetraenoate and its interaction with platelet-activating factor". Lipids 26 (12): 1184–8. doi:10.1007/bf02536528. PMID 1668115.
- ↑ 15.0 15.1 "Expression and function of OXE receptor, an eicosanoid receptor, in steroidogenic cells". Molecular and Cellular Endocrinology 371 (1–2): 71–8. May 2013. doi:10.1016/j.mce.2012.11.003. PMID 23159987.
- ↑ 16.0 16.1 16.2 "5-Oxo-ETE analogs and the proliferation of cancer cells". Biochimica et Biophysica Acta 1736 (3): 228–36. October 2005. doi:10.1016/j.bbalip.2005.08.009. PMID 16154383.
- ↑ "Relaxing effects of 5-oxo-ETE on human bronchi involve BK Ca channel activation". Prostaglandins & Other Lipid Mediators 83 (4): 311–9. June 2007. doi:10.1016/j.prostaglandins.2007.03.001. PMID 17499751.
- ↑ 18.0 18.1 "Stimulating properties of 5-oxo-eicosanoids for human monocytes: synergism with monocyte chemotactic protein-1 and -3". Journal of Immunology 157 (10): 4664–71. November 1996. PMID 8906847.
- ↑ "5-oxo-6,8,11,14-eicosatetraenoic acid induces the infiltration of granulocytes into human skin". The Journal of Allergy and Clinical Immunology 112 (4): 768–74. October 2003. doi:10.1016/S0091-6749(03)01888-8. PMID 14564360.
- ↑ Morin, C; Sirois, M; Echave, V; Gomes, M. M.; Rousseau, E (2007). "Relaxing effects of 5-oxo-ETE on human bronchi involve BK Ca channel activation". Prostaglandins & Other Lipid Mediators 83 (4): 311–9. doi:10.1016/j.prostaglandins.2007.03.001. PMID 17499751.
- ↑ "Enhanced formation of 5-oxo-6,8,11,14-eicosatetraenoic acid by cancer cells in response to oxidative stress, docosahexaenoic acid and neutrophil-derived 5-hydroxy-6,8,11,14-eicosatetraenoic acid". Carcinogenesis 32 (6): 822–8. June 2011. doi:10.1093/carcin/bgr044. PMID 21393477.
- ↑ "5-Oxo-6,8,11,14-eicosatetraenoic acid stimulates isotonic volume reduction of guinea pig jejunal crypt epithelial cells". The Journal of Pharmacology and Experimental Therapeutics 291 (2): 511–6. November 1999. PMID 10525065.
- ↑ 23.0 23.1 "5-Oxo-eicosatetraenoate is a broadly active, eosinophil-selective stimulus for human granulocytes". Journal of Immunology 157 (1): 336–42. July 1996. PMID 8683135.
- ↑ 24.0 24.1 "Chemical and biological characterization of oxo-eicosatetraenoic acids". Biochimica et Biophysica Acta 1201 (3): 505–15. December 1994. doi:10.1016/0304-4165(94)90083-3. PMID 7803484.
- ↑ "5-Oxo-eicosanoids and hematopoietic cytokines cooperate in stimulating neutrophil function and the mitogen-activated protein kinase pathway". The Journal of Biological Chemistry 271 (30): 17821–8. July 1996. doi:10.1074/jbc.271.30.17821. PMID 8663432.
- ↑ "Human neutrophil degranulation responses to nucleotides". Laboratory Investigation; A Journal of Technical Methods and Pathology 70 (6): 816–21. June 1994. PMID 8015286.
- ↑ "5-Oxo-6,8,11,14-eicosatetraenoic acid induces important eosinophil transmigration through basement membrane components: comparison of normal and asthmatic eosinophils". American Journal of Respiratory Cell and Molecular Biology 21 (1): 97–104. July 1999. doi:10.1165/ajrcmb.21.1.3517. PMID 10385597.
