Chemistry:Acidulant
From HandWiki
Acidulants are chemical compounds that give a tart, sour, or acidic flavor to foods or enhance the perceived sweetness of foods. Acidulants can also function as leavening agents and emulsifiers in some kinds of processed foods.[1] Though acidulants can lower pH they do differ from acidity regulators, which are food additives specifically intended to modify the stability of food or enzymes within it. Typical acidulants are acetic acid (e.g. in pickles) and citric acid. Many beverages, such as colas, contain phosphoric acid. Sour candies often are formulated with malic acid.[2] Other acidulants used in food production include: fumaric acid, tartaric acid, lactic acid and gluconic acid.[1]
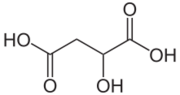
| Acid | Description | Formulation | pH |
|---|---|---|---|
| Acetic acid | Gives vinegar its sour taste and distinctive smell. | C2H4O2 | pH 3.91[3] |
| Ascorbic acid | Found in oranges and green peppers and gives a crisp, slightly sour taste, better known as vitamin C. | C6H8O6 | pH 3.59[3] |
| Citric acid | Found in citrus fruits and gives them their sour taste. | C6H8O7 | pH 3.24[3] |
| Fumaric acid | Found in bolete mushrooms, Icelandic moss and lichen, Not found in fruits, used as a substitute for citric and tartaric acid. Enhances flavor and sourness.[4] | C4H4O4 | pH 3.19[3] |
| Lactic acid | Found in various milk or fermented products and give them a rich tartness. | C3H6O3 | pH 3.51[3] |
| Malic acid | Found in apples and rhubarb and gives them their sour/tart taste. | C4H6O5 | pH 3.33[5] |
| Phosphoric acid | Used in some cola drinks to give an acidic taste. | H3PO4 | pH 3.06[3] |
| Tartaric acid | Found in grapes and wines and gives them a tart taste. Also called racemic acid. | C4H6O6 | pH 3.18[3] |
See also
- Food additive
- List of food additives
- Sour sanding
References
- ↑ 1.0 1.1 Berry, S.K.. (2001). Role of acidulants in food industry. Journal of Food Science and Technology. 38. 93-104.
- ↑ Erich Lück and Gert-Wolfhard von Rymon Lipinski "Foods, 3. Food Additives" in Ullmann's Encyclopedia of Industrial Chemistry, 2002, Wiley-VCH, Weinheim. doi: 10.1002/14356007.a11_561
- ↑ 3.0 3.1 3.2 3.3 3.4 3.5 3.6 Harald, Kalka. "pH of Common Acids and Bases". http://www.aqion.de/site/191#acids_name.
- ↑ Ipatenco, Sara (27 August 2014). "Fumaric Acid Foods". SF Gate. http://healthyeating.sfgate.com/fumaric-acid-foods-12220.html.
- ↑ "What is the pH?". Savetz Publishing. https://www.whatistheph.com/substance/Malic_Acid.
External links
- "Acidulants in Food", FAIA.org.UK.
 |
