Chemistry:Ambroxide
From HandWiki
Short description: Chemical compound
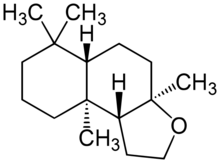
| |
| Names | |
|---|---|
| Preferred IUPAC name
(3aR,5aS,9aS,9bR)-3a,6,6,9a-Tetramethyldodecahydronaphtho[2,1-b]furan | |
| Other names
Ambrox (Firmenich)
Ambrofix (Givaudan) Ambroxan (Kao)[1] Ambermox Orcanox (3aR-(3aα,5aβ,9aα,9bβ))-Dodecahydro-3a,6,6,9a-tetra-methylnaphtho(2,1-b)furan; Naphtho(2,1-b)furan, dodecahydro-3a,6,6,9a-tetramethyl-,; 8α, 12-Oxido-13,14,15,16-tetranorlabdane; 1,5,5,9-Tetramethyl-13-oxatricyclo(8.3.0.0(4,9))tridecane | |
| Identifiers | |
| |
3D model (JSmol)
|
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| EC Number |
|
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| C16H28O | |
| Molar mass | 236.399 g·mol−1 |
| Density | 0.939 g/cm3 |
| Melting point | 75 °C (167 °F; 348 K) |
| Boiling point | 120 °C (248 °F; 393 K) (1.40 mm Hg) |
| insoluble | |
| Solubility in ethanol | soluble |
Refractive index (nD)
|
1.48 |
| Hazards | |
| Flash point | 161 °C (322 °F; 434 K) |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Ambroxide, widely known by the brand name Ambroxan, is a naturally occurring terpenoid and one of the key constituents responsible for the odor of ambergris. It is an autoxidation product of ambrein.[2] Ambroxide is used in perfumery for creating ambergris notes and as a fixative.[2] Small amounts (< 0.01 ppm) are used as a flavoring in food.[3]
Synthesis
Ambroxide is synthesized from sclareol, a component of the essential oil of clary sage.[4] Sclareol is oxidatively degraded to a lactone, which is hydrogenated to the corresponding diol.[5] The resulting compound is dehydrated to form ambroxide.[2]
References
- ↑ "Apply for a Trademark. Search a Trademark". http://www.trademarkia.com/ambroxan-78528957.html.
- ↑ 2.0 2.1 2.2 Karl-Georg Fahlbusch (2007), "Flavors and Fragrances", Ullmann's Encyclopedia of Industrial Chemistry (7th ed.), Wiley, p. 72
- ↑ George A. Burdock (2010), Fenaroli's Handbook of Flavor Ingredients (6th ed.), CRC Press, p. 1895
- ↑ Brian M Lawrence (2003). Essential Oils 1995-2000. Allured Pub.. ISBN 0-931710-94-4.
- ↑ Dub, Pavel A.; Gordon, John C. (2018). "The role of the metal-bound N–H functionality in Noyori-type molecular catalysts". Nature Reviews Chemistry 2 (12): 396–408. doi:10.1038/s41570-018-0049-z.
 |

