Chemistry:Antimycin A
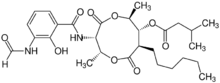
| |
| Names | |
|---|---|
| Preferred IUPAC name
(2R,3S,6S,7R,8R)-3-(3-Formamido-2-hydroxybenzamido)-8-hexyl-2,6-dimethyl-4,9-dioxo-1,5-dioxonan-7-yl 3-methylbutanoate | |
| Other names
Fintrol
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| MeSH | Antimycin+A |
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| C28H40N2O9 | |
| Molar mass | 548.633 g·mol−1 |
| Hazards | |
| Main hazards | Acute toxic |
| GHS pictograms | 
|
| GHS Signal word | Danger |
| H301, H311, H331 | |
| P261, P264, P270, P271, P280, P301+310, P302+352, P304+340, P311, P312, P321, P322, P330, P361, P363, P403+233, P405, P501 | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Antimycin A (more exactly Antimycin A1b) is a secondary metabolite produced by Streptomyces bacteria[1] and a member of a group of related compounds called antimycins. Antimycin A is classified as an extremely hazardous substance in the United States, as defined in Section 302 of the U.S. Emergency Planning and Community Right-to-Know Act (42 U.S.C. 11002), and is subject to strict reporting requirements by facilities which produce, store, or use it in significant quantities.[2]
Use
Antimycin A is the active ingredient in Fintrol, a chemical piscicide (fish poison) used in fisheries management.[citation needed]
Antimycin A was first discovered in 1945 and registered for use as a fish toxicant in 1960.[3] Fintrol ® is the only currently registered product containing Antimycin A and is classified as a restricted use pesticide because of its aquatic toxicity and requirement for highly specialized training in order to use it. In 1993, several toxicology studies were submitted to the United States Environmental Protection Agency yielding its toxicity.[3]
Fintrol is used primarily by federal and state governments in order to eliminate invasive species in an area where resident species are threatened. Antimycin A is added drop-wise in order to reach a concentration of 25 parts per billion.[3] These drip stations are typically used upstream in an area that is accessible to boats and traffic. In deeper bodies of water, a pump mechanism is used to disperse Antimycin A through a perforated hose stretching the length of the water column.[citation needed]
In aquaculture, Antimycin A is used as an agent to enhance catfish production via selective killing small and more sensitive species. When Antimycin A is added at 25 ppb it provides a complete kill. However at 10 ppb, Antimycin A is used as a selective killing agent to kill smaller or more sensitive species that may reduce the yield of commercial farming.
Products Containing Antimycin A can be registered providing they follow risk mitigation procedures.[3]
| Risk of Concern | Mitigation Measures |
|---|---|
| Exposure from consuming treated water |
|
| Exposure from consuming treated fish |
|
| Exposure from recreational activities in the treated water |
|
| Occupational Exposure |
|
| Ecological Risk Quotients for non-target species |
|
To date there has been no usage in human medicine, although its possibility as a chemotherapeutic was explored.[3]
Mechanism of action
Antimycin A is an inhibitor of cellular respiration, specifically oxidative phosphorylation. Antimycin A binds to the Qi site of cytochrome c reductase, inhibiting the reduction of ubiquinone to ubiquinol in the Qi site, thereby disrupting the Q-cycle of enzyme turn over. It also will cause the disruption of the entire electron transport chain. Due to this, there can be no production of ATP. Cytochrome c reductase is a central enzyme in the electron transport chain of oxidative phosphorylation.[4] The inhibition of this reaction disrupts the formation of the proton gradient across the inner membrane of the mitochondria. The production of ATP is subsequently inhibited, as protons are unable to flow through the ATP synthase complex in the absence of a proton gradient. This inhibition also results in the formation of the toxic free radical superoxide.[5] In presence of antimycin A the dependence of the superoxide production rate on oxygen level is hyperbolic.[6] In cultured cells at the background of mitochondrial respiration inhibition, the rate of superoxide production exceeds the cellular mechanisms to scavenge it, overwhelming the cell and leading to cell death.[citation needed]
It has also been found to inhibit the cyclic electron flow within photosynthetic systems along the proposed ferredoxin quinone reductase pathway.[7]
Although cyanide acts to block the electron transport chain, Antimycin A and cyanide act in different mechanisms. Cyanide binds a site in neighboring protein where iron normally binds, preventing oxygen from binding at all. This prevents cellular respiration completely leading to cell death.[8] Because Antimycin A binds to a specific protein in the electron transport chain, its toxicity can be highly species dependent because of subtle species specific differences in ubiquinol. This is why Fintrol can be used a selective killing agent in commercial farming.[citation needed]
Fungus-growing attine ants have been shown to use antimycins - produced by symbiotic Streptomyces bacteria - in their fungiculture, to inhibit non-cultivar (i.e. pathogenic) fungi.[9] One research group studying these symbiotic Streptomyces bacteria recently identified the biosynthetic gene cluster for antimycins, which was unknown despite the compounds themselves being identified 60 years ago. Antimycins are synthesised by a hybrid polyketide synthase (PKS)/non-ribosomal peptide synthase (NRPS).[10]
Toxicity
Lethal Doses
Lethal doses in fish and amphibian species [11]
| Species | LC50/24 hours exposure | LC50/96 hours exposure |
|---|---|---|
| Trout | 0.07 ppb | 0.04 ppb |
| Black Bullhead Catfish | 200 ppb | 45 ppb |
| Channel Catfish | >10 ppb | 9 ppb |
| Goldfish | 1 ppb | |
| Snails | 800 ppb | |
| Tiger salamander | >1080 ppb | |
| Tadpoles | 45 ppb | 10 ppb |
| Leopard Frog | 45 ppb | 10 ppb |
Lethal Doses in Mammals [12]
| Animal | LD50 mg/kg ingested |
|---|---|
| Rat | 28 |
| Mouse | 25 |
| Lamb | 1-5 |
| Dog | >5 |
| Rabbit | 10 |
Human Exposure and First Aid
Exposure to Treated Water: The effects of chronic, sub-lethal human exposure have estimated and extrapolated from murine (=pertaining to rodents) toxicology studies. Estimates in the literature have been determined using EPA risk assessment protocols.[13] Studies aimed at determining these levels found a concentration in mice where there is "No Observed Adverse Effect Level." From there, the EPA describes methods to determine a reference dose (RfD), the upper limit of the substance that can be consumed daily for the rest of one's life without any observable consequences. The RfD was determined to be 1.7 micrograms/kg/day.[14] For a grown adult, weighing around 70 kg, they can safely consume 2 liters of treated water at 60ppb.
Toxic effects may result from accidental ingestion of the material. Animal toxicology studies suggest that exposure to less than 40 grams of Antimycin A can result in serious adverse health effects to the individual.[15]
| Route of Exposure | Effect |
|---|---|
| Eye |
|
| Skin |
|
| Inhaled |
|
Treatment is focused on relieving symptoms and monitoring for respiratory distress, pulmonary edema, seizures, and shock.[15] Emesis after ingestion is not recommended for the potential of central nervous system depression.[16] Activated charcoal can be given as 240mL of water with 30g of charcoal.[16] The patient should be monitored for development of systemic symptoms and signs. After inhalation the patient should be moved to fresh air and monitored for bronchospasm, difficulty breathing, and respiratory distress. If needed, provide the patient with oxygen and secure an airway via tracheal intubation. Treat bronchospasm with inhaled beta2-adrenergic agonist and severe bronchospasm can be treated with systemic corticosteroids.[16]
References
- ↑ "Conditions influencing antimycin production by a Streptomyces species grown in chemically defined medium". Antimicrob. Agents Chemother. 1 (3): 274–6. March 1972. doi:10.1128/aac.1.3.274. PMID 4558141.
- ↑ 40 C.F.R.: Appendix A to Part 355—The List of Extremely Hazardous Substances and Their Threshold Planning Quantities (July 1, 2008 ed.). Government Printing Office. http://edocket.access.gpo.gov/cfr_2008/julqtr/pdf/40cfr355AppA.pdf. Retrieved October 29, 2011.
- ↑ 3.0 3.1 3.2 3.3 3.4 Caulkins, Peter. "Reregistration Eligibility Decision for Antimycin A". https://archive.epa.gov/pesticides/reregistration/web/pdf/antimycin-a.pdf.
- ↑ Kim, Hoeon; Esser, Lothar; Hossain, M. Bilayet; Xia, Di; Yu, Chang-An; Rizo, Josep; van der Helm, Dick; Deisenhofer, Johann (1999). "Structure of Antimycin A1, a Specific Electron Transfer Inhibitor of Ubiquinol−CytochromecOxidoreductase". Journal of the American Chemical Society 121 (20): 4902–4903. doi:10.1021/ja990190h. ISSN 0002-7863.
- ↑ "Oligomycin and antimycin A prevent nitric oxide–induced apoptosis by blocking cytochrome C leakage". J. Lab. Clin. Med. 143 (3): 143–51. March 2004. doi:10.1016/j.lab.2003.11.003. PMID 15007303.
- ↑ Stepanova, Anna; Konrad, Csaba; Manfredi, Giovanni; Springett, Roger; Ten, Vadim; Galkin, Alexander (2019). "The dependence of brain mitochondria reactive oxygen species production on oxygen level is linear, except when inhibited by antimycin A". Journal of Neurochemistry 148 (6): 731–745. doi:10.1111/jnc.14654. ISSN 1471-4159. PMID 30582748.
- ↑ Taira, Yoshichika (1 January 2013). "Antimycin A-like molecules inhibit cyclic electron transport around photosystem I in ruptured chloroplasts". FEBS Open Bio 3 (1): 406–410. doi:10.1016/j.fob.2013.09.007. PMID 24251103.
- ↑ Ott, Kevin. "Antimycin. A Brief Review of It's Chemistry, Environmental Fate, and Toxicology.". http://www.newmexicotu.org/Antimycin%20Summary.pdf.
- ↑ Schoenian, I. (2011). "Chemical basis of the synergism and antagonism in microbial communities in the nests of leaf-cutting ants". Proc Natl Acad Sci USA 108 (5): 1955–1960. doi:10.1073/pnas.1008441108. PMID 21245311. Bibcode: 2011PNAS..108.1955S.
- ↑ Yu, Jae-Hyuk; Seipke, Ryan F.; Barke, Jörg; Brearley, Charles; Hill, Lionel; Yu, Douglas W.; Goss, Rebecca J. M.; Hutchings, Matthew I. (2011). "A Single Streptomyces Symbiont Makes Multiple Antifungals to Support the Fungus Farming Ant Acromyrmex octospinosus". PLOS ONE 6 (8): e22028. doi:10.1371/journal.pone.0022028. ISSN 1932-6203. PMID 21857911. Bibcode: 2011PLoSO...622028S.
- ↑ Ott, Kevin. "Antimycin. A Brief Review of It's Chemistry, Environmental Fate, and Toxicology.". http://www.newmexicotu.org/Antimycin%20Summary.pdf.
- ↑ Ott, Kevin. "Antimycin. A Brief Review of It's Chemistry, Environmental Fate, and Toxicology.". http://www.newmexicotu.org/Antimycin%20Summary.pdf.
- ↑ Draft EIS, Flathead Westslope Cutthroat Trout Project. June 2004. p. Chapter 3.
- ↑ J. O. Kuhn, “Final Report. Acute Oral Toxicity Study in Rats”, Stillmeadow, Inc., Submitted to Aquabiotics Corp. (March 2001)
- ↑ 15.0 15.1 "Material Safety Data Sheet - Antimycin A". http://datasheets.scbt.com/sc-202467.pdf.
- ↑ 16.0 16.1 16.2 "Antimycin A". National Institutes of Health. https://toxnet.nlm.nih.gov/cgi-bin/sis/search/a?dbs+hsdb:@term+@DOCNO+6417.
 |

