Chemistry:Arsonium
From HandWiki
Short description: Ion
| |
| |
| Names | |
|---|---|
| Systematic IUPAC name
Arsonium | |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChemSpider | |
| 322800 | |
PubChem CID
|
|
| |
| |
| Properties | |
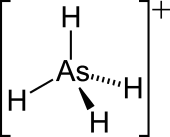
| AsH+4 | |
| Molar mass | 78.954 g·mol−1 |
| Conjugate base | Arsine |
| Structure | |
| Tetrahedral | |
| Related compounds | |
Related compounds
|
ammonium phosphonium |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Tracking categories (test):
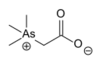
The arsonium cation is a positively charged polyatomic ion with the chemical formula AsH+4. An arsonium salt is a salt containing either the arsonium (AsH+4) cation, such as arsonium bromide (AsH+4Br−) and arsonium iodide (AsH+4I−), which can be synthesized by reacting arsine with hydrogen bromide or hydrogen iodide.[1] Or more commonly, as organic derivative such as the quaternary arsonium salts Ph4As+Cl− (CAS: 123334-18-9 , hydrate form) and the zwitterionic compound arsenobetaine.
References
- ↑ Muñoz‐Hernández, M. Á. (2006). Arsenic: Inorganic Chemistry. Encyclopedia of Inorganic Chemistry. pp 4. DOI: 10.1002/0470862106.ia013
 |