Chemistry:Asarone
| |||
| Names | |||
|---|---|---|---|
| IUPAC names
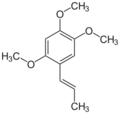
1,2,4-Trimethoxy-5-[(E)-prop-1-enyl]benzene (α)
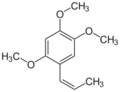
1,2,4-Trimethoxy-5-[(Z)-prop-1-enyl]benzene (β) | |||
| Other names
alpha-Azaron
cis-Isoelemicin 2,4,5-Trimethoxyphenyl-2-propene | |||
| Identifiers | |||
3D model (JSmol)
|
| ||
| ChEMBL | |||
| ChemSpider | |||
PubChem CID
|
|||
| UNII |
| ||
| |||
| |||
| Properties | |||
| C12H16O3 | |||
| Molar mass | 208.257 g·mol−1 | ||
| Appearance | Colorless solid | ||
| Density | α: 1.028 g/cm−3 [1] | ||
| Melting point | 62 to 63 °C (144 to 145 °F; 335 to 336 K)[2] (α) | ||
| Boiling point | 296 °C (565 °F; 569 K)[2] (α) | ||
| Insoluble | |||
| -131.4·10−6 cm3/mol | |||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |||
| Infobox references | |||
Asarone is chemical compound of the phenylpropanoid class found in certain plants such as Acorus and Asarum.[2] There are two isomers, α (or trans) and β (or cis).[3] As a volatile fragrance oil, it is used in killing pests and bacteria.[4]
Pharmacology
The main clinical symptom of asarone is prolonged vomiting that sometimes lasted more than 15 hours. Asarone is not metabolized to trimethoxyamphetamine as has been claimed by online vendors.[5]
The Council of Europe Committee of Experts on Flavouring Substances concluded that β-asarone is clearly carcinogenic[6] and has proposed limits for its concentration in flavorings such as bitters made from Acorus calamus (sweet flag).[7]
β-Asarone exhibits anti-fungal activity by inhibiting ergosterol biosynthesis in Aspergillus niger.[8] However, the toxicity and carcinogenicity of asarone means that it may be difficult to develop any practical medication based on it.[9]
See also
Notes and references
- ↑ Data for α-Asarone at ChemSpider
- ↑ 2.0 2.1 2.2 "Asarone". The Merck Index. 14th edition. Merck Research Laboratories. 2006. pp. 135. ISBN 978-0-911910-00-1.
- ↑ Beta asarone has CAS# 5273-86-9
- ↑ "Antimicrobial activity of Acorus calamus (L.) rhizome and leaf extract". Acta Biol. Szeg. 53 (1): 45–49. 2009.
- ↑ "Bioanalytical investigation of asarone in connection with Acorus calamus oil intoxications". J Anal Toxicol 33 (9): 604–9. 2009. doi:10.1093/jat/33.9.604. PMID 20040135.
- ↑ Cartus, Alexander T.; Stegmüller, Simone; Simson, Nadine; Wahl, Andrea; Neef, Sylvia; Kelm, Harald; Schrenk, Dieter (2015-08-26). "Hepatic Metabolism of Carcinogenic β-Asarone". Chemical Research in Toxicology 28 (9): 1760–1773. doi:10.1021/acs.chemrestox.5b00223. ISSN 0893-228X. PMID 26273788.
- ↑ Opinion of the Scientific Committee on Food on the presence of β-asarone in flavourings and other food ingredients with flavouring properties. European Commission Scientific Committee on Food. 8 January 2002. http://ec.europa.eu/food/fs/sc/scf/out111_en.pdf.
- ↑ Venkatesan, Ramya; Karuppiah, Prakash Shyam; Arumugam, Gnanamani; Balamuthu, Kadalmani (2017-11-10). "β-Asarone Exhibits Antifungal Activity by Inhibiting Ergosterol Biosynthesis in Aspergillus niger ATCC 16888" (in en). Proceedings of the National Academy of Sciences, India Section B: Biological Sciences 89: 173–184. doi:10.1007/s40011-017-0930-4. ISSN 0369-8211.
- ↑ Perrett, Sheena; Whitfield, Philip J. (1995). "Anthelmintic and pesticidal activity of Acorus gramineus (Araceae) is associated with phenylpropanoid asarones". Phytotherapy Research 9 (6): 405. doi:10.1002/ptr.2650090604.
 |